One-on-One Interview with Rella P. Christensen, RDH, Ph.D.
Dr. Rella Christensen is a well-known researcher and lecturer in clinical dentistry. She has directed many scientific studies, authored research abstracts and received numerous honors. Dr. Christensen cofounded the nonprofit Clinicians Report (CR) Foundation (formerly known as Clinical Research Associates [CRA]), serving as its director and lead researcher for 27 years. Currently, she is the team leader of Technologies in Restoratives and Caries (TRAC) Research, which conducts controlled clinical studies within practice environments to investigate treatment outcomes as they occur in the hands of working dentists. In this interview, Dr. Christensen discusses the present and future endeavors of TRAC Research, the search for the “Holy Grail” of restorative dental materials, and the new frontier of chemical treatment for dental caries.
DR. NEIL PARK: You’ve established a unique organization for clinical evaluation. Can you tell us a little bit about how the CR Foundation® developed and how it runs today?
DR. RELLA CHRISTENSEN: In 1976, my husband, Dr. Gordon Christensen, had the idea that dental clinicians could and should formally evaluate the products they use clinically. Up to that time, product evaluation had been in the hands of various people who were hired for that purpose within and outside of universities. The organization started with four study clubs that Gordon was mentoring at that time — they were in four different states and were made up of more than 120 dentists.
The idea was to use the products, evaluate their use characteristics, and follow their clinical service over time, both clinically and scientifically. As more and more products were brought into the system, there was a need for more and more evaluators. We also realized that the most real-world evaluation would be gleaned from a very diverse group of clinicians, a group that reflected diversity not only in their backgrounds, training and years out of school, but also in their skill levels and interests.
We secured a list from the American Dental Association, which back in the late ’70s included most of the practicing dentists in the U.S., and from that list we were able to determine the number of dentists practicing in the various regions, states and localities. We constructed for each state a group of dentists interested in evaluating products that proportionally reflected the percentage of dentists in each state and locality. For example, California would have more evaluators than Nevada or Idaho.
NP: One of the hallmarks of your research organization has been that you stay independent of manufacturers. But it must be a challenge to afford to do these kinds of activities without funding from corporations.
RC: We made the decision from the very beginning to have absolute objectivity. In order to do that, we felt we must fund ourselves and not rely on any type of outside funding. The thinking was that the dentists, laboratory technicians, dental hygienists and assistants would be willing to provide both the skills and the money to accomplish this. This meant we all needed to volunteer. Everyone volunteers their expertise, time and services. And that includes Gordon and myself. That, of course, greatly reduces the overhead.
We made the decision from the very beginning to have absolute objectivity. In order to do that, we felt we must fund ourselves and not rely on any type of outside funding.
NP: Sure, but even if all of the clinicians volunteer their time, it still takes money to run an organization.
RC: Monetary income is generated in two ways: through the sale of a monthly publication of the results of the work (Gordon J. Christensen Clinicians Report®), and through providing courses that review the products and techniques studied over the past 12 months (CR “Dentistry Update” courses). One-hundred percent of the net income generated goes directly to the research projects.
NP: So you’re producing these publications that summarize findings while you’re doing lectures that bring the clinicians up to date on what’s going on in the industry, and then you’re using those funds to fund further research. It’s a very virtuous cycle, from the way I see it.
RC: It is altruistic, but it has worked well over the years, and I think the nonprofit foundation orientation has resulted in the best possible objectivity. We have found that dental clinicians are more than willing to help identify products and techniques that help them deliver better treatment for their patients.
NP: What is the relationship between the CR Foundation and TRAC Research?
RC: TRAC Research is the human-studies branch of what’s now the CR Foundation. In 2004, when Gordon and I came back from serving a two-year mission for our church, I thought it was the right time to turn over the administration of what was then CRA to someone else. I had served in that position for 27 years and put in place the policies and procedures that governed the day-to-day operation, I had also overseen and participated in all the research work. I was ready for a change. The organization was renamed to show the change in leadership. I then had the opportunity to do what I was trained to do and what I really wanted to do — basic research in oral microbiology and long-term evaluation of restorative materials.
TRAC Research performs large human-research studies. My Ph.D. is in physiology with an emphasis on microbiology. This combination is ideal for working with diseases that result from shifts in normal flora. We set up a highly specialized and sophisticated lab needed to do this work, and have been very busy developing the necessary techniques and procedures.
We study what lives in the oral cavity and what is put into the oral cavity, a continuum that ranges from dental caries through dental materials. We travel with the essentials of our lab to various dentists’ offices, where we secure samples and data while they are operating on patients.
We study what lives in the oral cavity and what is put into the oral cavity, a continuum that ranges from dental caries through dental materials.
NP: Very interesting. So with all of this clinical data, especially on restorative materials, that you’ve been collecting over the years, and all of this microbiological data that you’ve been gathering from human subjects, can you tell us about some of the most interesting findings that you’ve uncovered?
RC: We finally have collected sufficient data to begin reporting. It took us about 10 years to get the things we needed in our lab, and to understand what we needed to do and then learn how to do it. One of the most important things we’ve learned is the incredible ability of oral microbes to survive and to multiply, even under the most adverse conditions. We can now prove that microbes are able to continue to survive under restorations and continue their process of tooth destruction. Today we have no way for clinicians to monitor this continuing activity. Patients generally do not experience pain because the process is anaerobic and moves slowly, so the tooth adapts. What clinicians can observe from the outside is not indicative of what is occurring at the lesion front. Yet despite the lack of symptoms, we’ve come to realize that many lesions actually have progressed to the point that they connect with the dental pulp, and that establishes an interesting oral-systemic connection. We haven’t yet determined what happens once the microbes enter the pulp. We don’t know if they’re met by the immune cells and eliminated there, or if they’re able to gain access to the bloodstream and the body. A few of the interesting things we have good visual, numerical and DNA data to prove include:
- All finished preparations are highly contaminated.
- None of our dental materials provide a perfect seal — none — despite claims.
- There are no infected and affected layers — microbes penetrate all the way to the advancing front.
- Many organisms are involved in the process, and they can differ from patient to patient.
- Organisms remain viable under restorations and sealants.
- Healthy dentin is sterile.
NP: So it sounds like the clinician’s ability to interfere with the disease process might be overrated?
RC: Well, the conventional removal of the most densely infected dentin is definitely helpful, but to really stop the pathology, more is needed. We knew from our microbiology training that the organisms were what is called “facultative.” That means they have evolved to survive with two types of physiologies: either in the presence of oxygen, or without oxygen. They can switch back and forth from an aerobic to an anaerobic physiology as conditions warrant the need.
NP: Is the anaerobic form associated with more disruptive disease processes?
RC: Anaerobically, the organisms’ progress within the tooth may be slower, but anaerobic physiology allows the organisms to survive under conditions that I don’t think we considered them capable of surviving before. Some have thought the organisms survived on the nutrients that patients eat and that when covered with restorative materials, they are cut off from their nutrient source and cannot survive. We didn’t think of the tooth as the substrate. If the tooth is the substrate, the organisms would have the ability to survive anaerobically within the tooth quite well for a long time. We have found that when we culture both aerobically and anaerobically — which we always do with all samples we take — we have far more diversity and growth in the anaerobic state than in the aerobic state.
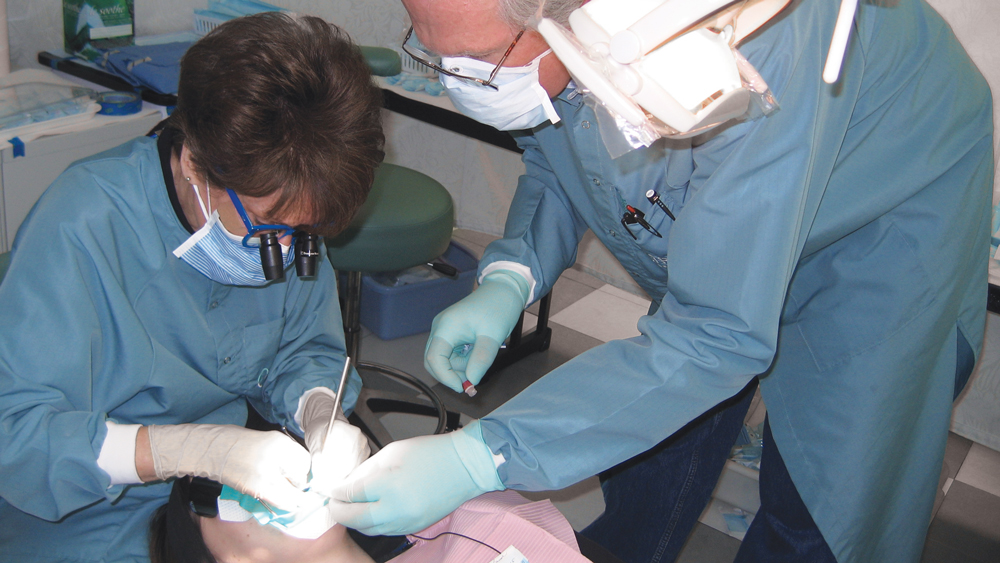
Dr. Christensen and Brad Ploeger, a virologist with TRAC Research, performing a sterile harvest of microorganisms from an active caries lesion in an adult patient.
NP: So do you think that the future of our treatment regimens will include more chemotherapeutic-type agents?
RC: Absolutely. That is what we have been working on — chemical treatment of caries. In lieu of cutting the tooth, or in addition to more conservative cutting, we propose stopping the lesion progression by the use of chemicals. We have already proposed use of GLUMA® desensitizing agent (Heraeus Kulzer; South Bend; Ind.) for disinfection of all completed preparations before moving into the restorative steps in order to kill the microbes that are always abundantly present in finished preps. This chemical is composed of 5% glutaraldehyde and 35% hydroxyethyl methacrylate (HEMA). As such, it provides excellent disinfection and penetrates the smear layer and into the dentin while also providing desensitization and modestly enhancing bond strengths and bond longevity over time.
NP: That’s great information. Do you have any studies currently underway that might greatly affect the way dentists practice, or change the way we think about a trusted material or procedure?
RC: In the area of restorative dentistry, we have been looking since 1976 for something that acts like cast gold but is white. We have seven-year clinical data now that suggests that the original full-strength BruxZir® Solid Zirconia may fulfill that search — and a close second is IPS e.max® CAD (Ivoclar Vivadent; Amherst, N.Y.) when it is handled properly.
NP: Any idea of how many materials you’ve looked at in the quest for this Holy Grail?
RC: If we count the 10 new materials we’re looking at right now, it would bring the total to 128 over a 41-year period. Seven years ago we started a study of the original full-strength BruxZir Solid Zirconia alongside IPS e.max CAD, placing these two materials as full crowns on molars. We’re now well into the seventh year of that study, and both of these materials have performed exceptionally well. We have had 100 percent survival of the BruxZir restorations and 99 percent survival of the IPS e.max CAD in seven years. These two materials have not shown the cracks, chips and fractures typical of ceramics, or the washout seen with polymers and some other white materials. With the original full-strength BruxZir Solid Zirconia, we were initially skeptical, but it has proven itself clinically to be worth our attention.
NP: My generation of dentists has certainly been through some white materials that have disappointed us.
RC: That’s very true. We designed our work to study the questions and accusations against the use of full-contour zirconia, such as how it would affect the occlusal mechanism, including the joint, the muscles, the periodontal membrane, and so on. We’ve watched very carefully. Believe it or not, we’ve actually located and measured every facet on every BruxZir and IPS e.max CAD restoration, along with the one or two teeth opposing them. We have mathematically calculated facet progression over three years. We also watched the joint and soft tissue closely. We found that BruxZir Solid Zirconia itself is worn by every type of opposing material, including enamel. This was not what we expected. In fact, we’ve made photographs of the facets on the BruxZir crowns to show other researchers and clinicians who have been very skeptical of that finding.
NP: And it’s a natural skepticism, because you would think a material with such great flexural strength would be kind of a mismatch for other materials that go in the mouth.
RC: Actually, flexural strength may not be the best measure of projected wear of opposing dentition. Other properties such as hardness and particle size and particle distribution may be the decisive factors. We’ve been interested in the way the BruxZir formulation is manufactured in a colloidal form, and what exactly that means, because it is one of only two zirconia formulations worldwide right now that uses this method, which allows it to avoid use of binders and maintain a high level of purity. We are one year into a study where we’re examining eight zirconia materials coming from diverse locations such as Asia, Europe, and Newport Beach and San Clemente, California. We’re looking at the variety of ways these products are being handled during processing. After a couple of years monitoring the clinical performance of these products, we’ll know more about how the different methods play out. We will also know how that performance compares with full-strength BruxZir Solid Zirconia.
Flexural strength may not be the best measure of projected wear of opposing dentition. Other properties such as hardness and particle size and particle distribution may be the decisive factors.
NP: I think that’s an important point that a lot of dentists may not be aware of: Different formulations of zirconia can differ greatly in their physical properties.
RC: We’ve tried to tell the profession that different zirconia formulations can differ in a lot of ways: particle size, particle size distribution, purity, the type and amount of additives such as oxides and binders, as well as radioactivity. Even the methods companies use to form their disks can make a difference in how the zirconia performs clinically.
Many dentists still do not realize that the newer translucent zirconias have a formulation that is very different from the original full-strength BruxZir Solid Zirconia. The differences result in a significant drop in flexural strength and change in the transformation toughening, the characteristic that makes the restorations so resistant to oral stresses. The clinicians also may not realize that coloring ions further reduce strength. So formulation of zirconia is an important issue clinically.
Another issue you never hear about is radioactivity and how much of it is remaining in these various products. Zirconia occurs in nature closely associated with several radioactive materials, and they’re very, very hard to separate out. In fact, they can’t totally remove all the hafnium present. Most people have never even heard of hafnium, let alone realize that it’s there. Some of the products coming from unknown sources could possibly even be dangerous in the amounts of radioactivity present. For this reason, we suggest that labs and dentists use zirconia from well-known companies to guard against problems down the road.
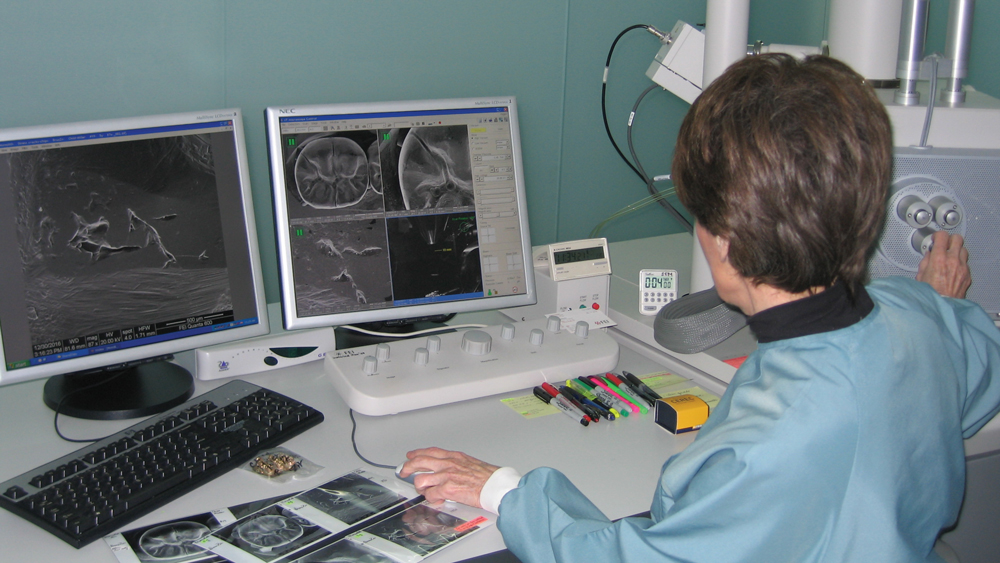
Dr. Christensen at her scanning electron microscope, making images to document a problem with a restoration under study.
NP: Wow. Regarding the BruxZir material, do you feel the smaller particles and the absence of binders are responsible for the physical characteristics that you’ve noted?
RC: We think so, yes, but we’ll know more as we compare the original BruxZir to some of the zirconias handled differently today. We want to see how the other methods and innovations to produce translucence and coloring affect handling and clinical performance. We have already reported months ago that the translucent zirconias need more gentle chairside handling by the dentists during occlusal adjustments and they need more careful attention to tooth reduction during tooth preparation. The study of BruxZir Solid Zirconia and IPS e.max CAD is ongoing. We’ll continue to monitor these two materials as long as they’re relevant and we can still locate the patients. These two materials have now become the controls as we study new brands entering the market. We think performance of the newer materials should equal or exceed the original full-strength BruxZir Solid Zirconia or IPS e.max CAD, on which we now have well-documented clinical data at seven years.
NP: Physical characteristics are very important, but, of course, you have to produce something that fits and has good esthetics as well. Have you also looked at these variables?
RC: You know, BruxZir Solid Zirconia restorations were never promoted as gorgeous, but were said to have adequate esthetics to accompany its “brawn.” We’ve wondered what’s made BruxZir Solid Zirconia so successful. I mean, about 14 million units have been prescribed in seven years — that’s an amazing acceptance rate in the absence of clinical data. We think some factors might have been the time in which it was introduced as well as its pricing and name. In 2009, full-strength BruxZir crowns were introduced for about $100 a unit when dentistry was getting squeezed by the recession.
We think the name “BruxZir” was important, too, because it sent a strong message to clinicians that this ceramic would withstand bruxing and clenching. And fortunately for Glidewell Dental, the product has withstood these challenges well, while a lot of other materials haven’t. We think BruxZir Solid Zirconia has been an amazing and continuing success. According to our seven-year scanning electron microscope data, BruxZir restorations still look just like they did the day they were seated. That’s pretty impressive performance for an all-ceramic at seven years.
According to our seven-year scanning electron microscope data, BruxZir restorations still look just like they did the day they were seated. That’s pretty impressive performance for an all-ceramic at seven years.
NP: What do you think are some of the unmet needs for clinicians currently in practice? As a company with an active R&D program, Glidewell Dental is always looking for the next great product. Is there a missing material, a missing procedure, that would greatly benefit our colleagues?
RC: There is, and it’s one that might actually be applicable to a large lab producing thousands of units. In my opinion, the best way to control dental caries is to change the oral environment — rather than trying to kill the bacteria. I would like to see a material that could control the oral pH, regardless of what the patient ate or drank.
NP: You’re thinking restorative material?
RC: It could be, but my idea is to veneer the molars, on the facial and lingual to maximize the surface area, with a material that has buffer capacity. When you change the oral pH by ingesting sugar or low-pH foods or drinks, it would bring the pH back to around 6.5 to 7.5 — somewhere in that range. Think about it: If the pH didn’t drop, what would cause the tooth to demineralize?
NP: And all of the bacteria in the biofilm that demineralize tooth structure, they do it by lowering the pH, right? That’s the requirement?
RC: I believe oral pH is the key. I would not limit use of this material with buffer capacity to areas needing restoration. Instead I would call it and use it as a preventive material. And a lab like Glidewell Laboratories could develop something like that. It could be attached almost like false fingernails. Dentists would probably have to replenish the substance with buffer capacity as it degrades over time in order to maintain the pH control.

Dr. Christensen working with the TRAC Research team to collect data on dental restorative material performance in long-term controlled clinical trials.
NP: So, it’s some kind of material that would exert a constant long-term buffering effect on pH. Are there any possible ill effects of preventing the pH from getting too low?
RC: We know there are ill effects as a result of allowing the pH to drop to low levels for extended time periods. The question is whether there are ill effects from holding it steady and near neutral. This question is something we need to study longer over time. We know the organism population shifts if the pH is maintained in a near neutral range. We’ve tested this in oral cavities where the teeth were polished carefully on a weekly basis. We actually got a shift in the ratio of organisms that made up the total flora. I liked what I saw because you don’t wipe out the species that like low pH, but instead you reduce their numbers while other less-detrimental species increase in number in response to the more favorable environment for them.
But, you know, people eat awful things. They can’t just buy a soda and drink it down. Instead, they buy one that’s massive and sip on it all day, which keeps the pH low for hours. Enamel begins to dissolve at about 5.5. A few years ago, we worked with a group of children 9–18 years old in orthodontic treatment, and it was amazing to us that out of this group of 320 kids, many of them lived on sugary cereals because they could just pour it out, add a little bit of milk, and eat it. A lot of working moms aren’t cooking much, so the kids are turning to sugary cereals because of its availability and taste. These habits are not conducive to optimal oral health or overall health.
NP: So you’re doing research on pH control. Anything you’d like to tell our readers?
RC: I don’t have any specific product or regimen to suggest yet. We’re not trying to develop a product; I am trying to suggest a concept. We evaluate products, so if I was trying to develop a product, I feel it would almost be a conflict of interest. But I would love to get someone interested in going down the pH-control route. The product I have in mind would have to be tasteless and odorless, yet have buffering capacity. It would also probably need to be replenished — or “recharged,” as we say in dentistry — because it would be left in the oral cavity 24/7. It would be impossible for the patient to forget to use it, as in the case of a dentifrice or a rinse.
NP: Right. Maybe we just add it to the breakfast cereals.
RC: I would hope for something better than a cereal addition. I hate to encourage eating sugary cereals routinely. I see this as a professionally placed therapeutic preventive product.
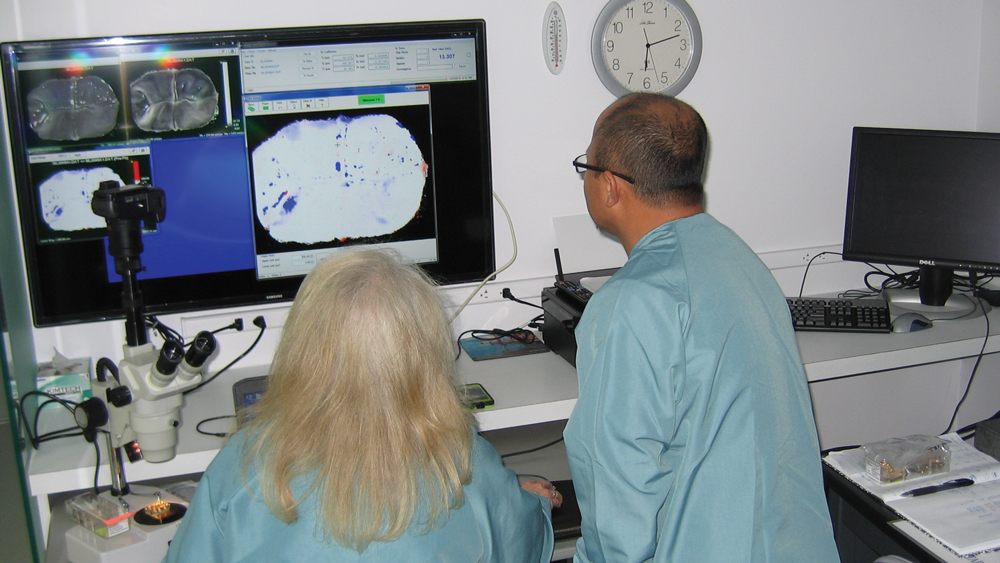
The CRA Measurement System is used to quantify wear and measure depth of chips in microns.
NP: So, with so much important research being conducted and published, what’s the best way for general dentists to learn about this research so they can apply it to their clinical practices?
RC: Today it seems like people don’t read hard copy materials, but they do spend time on the internet. Gordon has just started an International Study Club where he goes online on a weekly basis and spends about five to 10 minutes answering the most frequently asked question of that week. He gleans the questions from more than 50,000 a year that are submitted to the CR Foundation via email, snail mail and telephone. Watching something on the internet any time you choose is very easy, and getting an answer to a very timely question could save a clinician a lot of grief.
NP: How would our readers find out about this?
RC: Just go to Gordon’s website, pccdental.com. Reading the monthly Gordon J. Christensen Clinicians Report electronically or in hard copy is another easy, fast way to get objective, timely information.
There are myriad other sources where dentists can not only keep up, but also become aware of a variety of theories and opinions. A lot of dentists don’t enjoy reading basic research, such as charts, graphs and statistics. They like to know the bottom line: “What did you find out? What should I use?” The Gordon J. Christensen Clinicians Report and International Study Club report are in that form. If you want the materials and methods and basic science, just ask and this information is available.
A lot of dentists don’t enjoy reading basic research, such as charts, graphs and statistics. They like to know the bottom line.
NP: Exactly. And, of course, the CR Report has always been a resource.
RC: Well, maybe not always, but for the last 41 years the Clinical Research Associates/Gordon J. Christensen Clinicians Report has been available, and is still moving forward, conducting actual clinical research (not just opinions and theories) on just about everything dental clinicians use in practice.
NP: Where do you see restorative dentistry heading in the next five to 10 years?
RC: I see two major thrusts. One is more automation. Glidewell Dental is doing interesting things that eliminate the human element. We hope that more automation in the industry will lead to improved consistency.
The other thrust is the chemical treatment of caries. Recently, we have been studying silver diamine fluoride for chemical treatment of dental caries. This treatment is projected for children and senior citizens, and it is now being practiced as a viable treatment at UC San Francisco. The chemical is simply painted onto lesions to at least slow their progress without use of local anesthetic, handpiece or dentist. We think some disinfectant yet to come forward will probably become very important on a public health basis for treatment of dental caries, both nationally and internationally.
NP: So we’ll look forward to some very, very potent new tools in preventive dentistry.

Dr. Christensen working with an expert on ion transfer monitoring in an attempt to validate this concept using pediatric teeth exfoliated several years after treatment.
RC: Yes. To my knowledge we have not used disinfectants a lot in the U.S. in connection with dental caries treatment. Interestingly they’ve been used for years in Japan and other countries around the world. Silver diamine fluoride is not new. There is a lot of data on its use over many years in Japan.
NP: It sounds like a very exciting development to look forward to. Any future plans for TRAC Research and the CR Foundation that you can share?
RC: We plan on continuing as we have been this past 41 years — performing clinical and basic science testing of all types of dental products and techniques. Gordon and I are not the only people working for the Clinicians Report Foundation. There are about 50 people on-site and about 450 in dental offices in many countries. TRAC Research’s large-scale human studies will certainly continue. As far as CR as a whole, there will be lots of exciting new things as the leadership moves forward.
NP: I want to thank you for all of your contributions to clinical dentistry, and for sharing your knowledge and research.
RC: Thank you for the opportunity.




