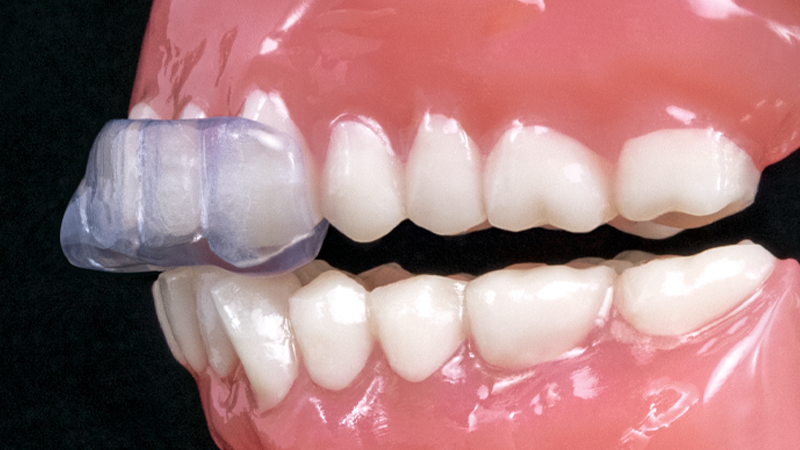
- Covers the anterior teeth and can be prescribed for the upper or lower arch
- Reduces clenching forces
- Cleared by the FDA for the prevention of migraine and tension-type headaches


NTI-tss Plus®
*Price is per device and does not include shipping or applicable taxes.
The NTI-tss Plus® is an anterior disclusion device proven effective at preventing tension headaches and migraines. Its patented design keeps the posterior teeth and canines out of occlusion, which reduces clenching intensity and protects against tooth damage and bone loss.
The Migraine Relief Your Patients Have Been Waiting For
The NTI-tss Plus (Nociceptive Trigeminal Inhibition Tension Suppression System) serves as a drug-free alternative treatment to migraine pain. With over 30 million people in the U.S. suffering from migraines, dental clinicians have an opportunity to help their patients get out of pain.1 The device has been cleared by the FDA for the prevention of migraine and tension-type headaches and has been shown to reduce clenching forces. In one study, 82% of migraine sufferers experienced a 77% reduction in migraine events when using the NTI-tss Plus.2
The new NTI OmniSplint® similarly minimizes clenching and bruxing intensity but does so through a full-arch design. By covering both arches, the NTI OmniSplint provides patients with a comfortable solution for their migraines or tension headaches while eliminating concerns of aspiration, supereruption of the posterior teeth, or long-term use.
NTI devices are especially valuable for patients who are pregnant or nursing, or those with medical complications who cannot accept pharmacological migraine treatments. An NTI device may be indicated for patients who experience possible symptoms of tension headaches, migraines, TMD, tooth wear from clenching, or muscle pain associated with parafunction.
NTI-tss Plus is a registered trademark of Boyd Research, Inc. NTI OmniSplint is a registered trademark of James Boyd.
Reduces Clenching and Migraine Frequency
The NTI-tss Plus and the NTI OmniSplint have been shown to reduce clenching forces. When wearing the NTI-tss Plus regularly, 82% of migraine sufferers experienced a 77% reduction in migraine events.3
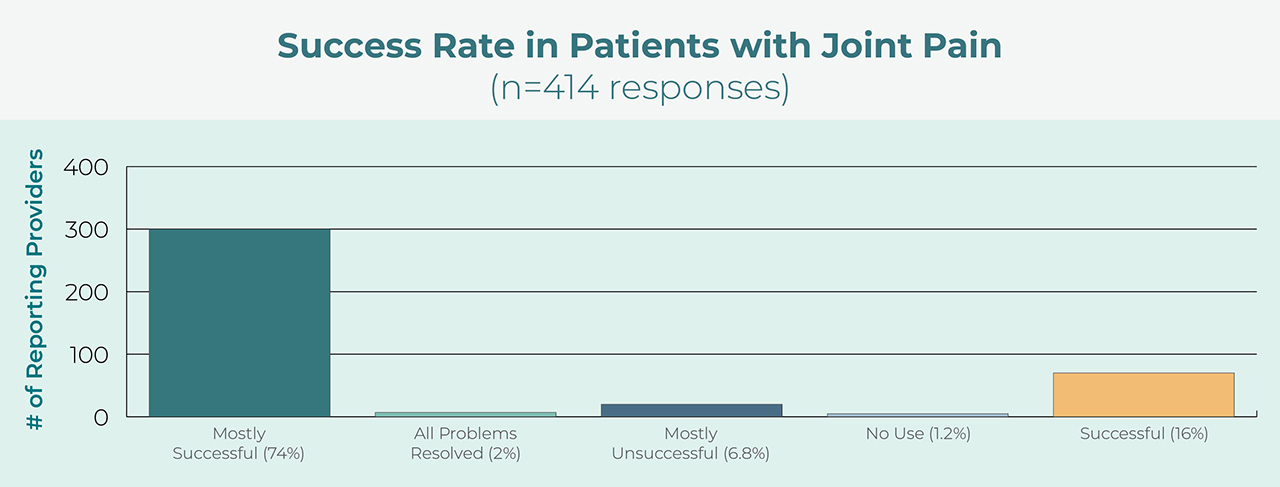
High Success Rate
Users report a high degree of success with the appliance for treatment of orofacial pain.4 Additionally, prescribers use the device for both pain control and protection of teeth and restorations.
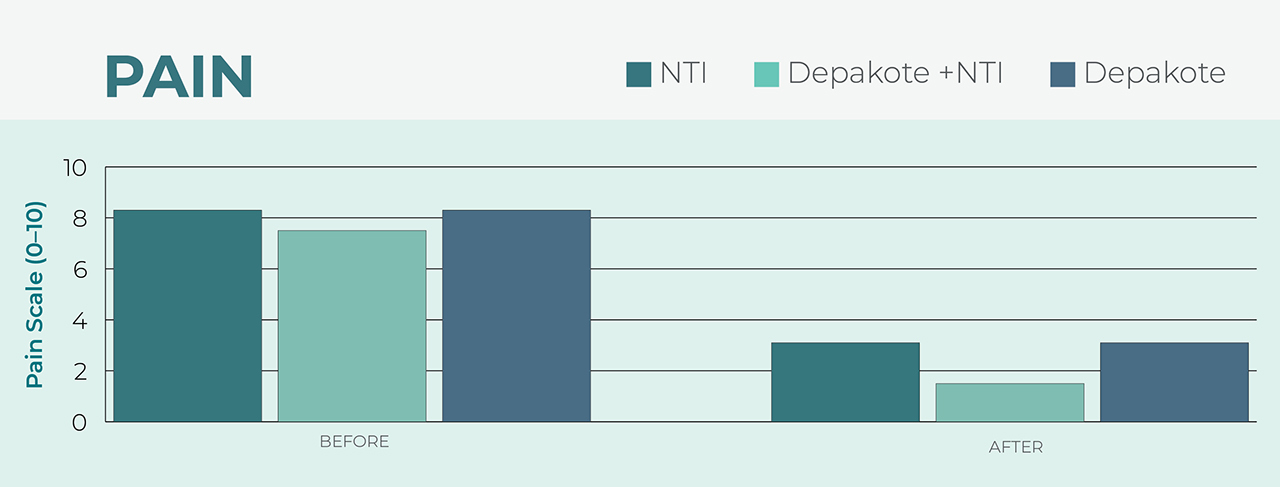
Alternative to Pharmacological Treatment
After using the NTI, wearers reported the same reduced pain level as individuals who used a common migraine medication, Depakote®.5 The benefit of prescribing an NTI over a pharmacological treatment is the device’s lack of harmful side effects that may manifest in patients taking prescription medications.
Depakote is a registered trademark of Sanofi Corporation.
Prescribing an NTI device for your patient is more than just offering a trusted solution to their problems — it’s about securing a patient for life. When you relieve someone of their chronic migraine pain, you dramatically transform their life for the better, and that’s something every dentist should want to be a part of.
Indications
Indicated for patients who experience possible symptoms of tension-type headaches, migraines, TMD, tooth wear from clenching, or muscle pain associated with parafunction.
Material Composition
Biocompatible, light-cured resin
Types
- NTI-tss Plus
- NTI OmniSplint
In-Lab Working Times
4 working days in lab
Patient Cleaning Instructions
Run the device under warm water each time you remove the device. Do not use toothpaste, mouthwash or alcohol-based products for soaking or cleaning. Do not boil.
Pricing
|
NTI-tss Plus®(buy 1)
|
$131.00 |
|
Glidewell Clinical Twinpak(buy 2)
|
$242.001 |
Glidewell Clinical Twinpak is valid for two appliances for the same case.
Pricing is subject to change and does not include shipping or applicable taxes.
Policies & Warranty
NO-FAULT REMAKE POLICY: Glidewell is pleased to process all remakes or adjustments at no additional charge if requested within the warranty period and accompanied by the return of the original appliance.
LIMITED WARRANTY/LIMITATION OF LIABILITY. Glidewell (“the lab”) warrants that all dental devices (a “device”) are made according to your specification and approval in the belief that the device will be useful and MAKES NO OTHER WARRANTIES INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. Subject to the return of a device that is placed and then fails, the lab will repair or replace the device without charge for the cost of materials and workmanship or refund the original price paid, at the lab’s option, for up to 6 months for the NTI-tss Plus and the NTI OmniSplint.
Clinical Tips
References
- ^ Migraine Research Foundation. (2021, January 15). Migraine Facts. Migraine Research Foundation. migraineresearchfoundation.org/about-migraine/migraine-facts/.
- ^ Shankland WE 2nd. Migraine and tension-type headache reduction through pericranial muscular suppression: a preliminary report. Cranio. 2001;19(4):269-278.
- ^ Shankland WE 2nd. Migraine and tension-type headache reduction through pericranial muscular suppression: a preliminary report. Cranio. 2001;19(4):269-278.
- ^ Blumenfeld, Andrew, et al. “Patterns of Use for an Enhanced Nociceptive Trigeminal Inhibitory Splint.” Inside Dentistry, Aegis Dental Network, 1 Dec. 2011, www.aegisdentalnetwork.com/id/2011/12/patterns-of-use-for-an-enhanced-nociceptive-trigeminal-inhibitory-splint.
- ^ Blumenfeld, A., MD, Tarzemany, R., DDS, & Beladimoghaddam, N., MD. (2011). Combination Therapy: Depakote with an NTI Clenching Reduction Dental Splint for Prophylactic Treatment of Primary Headache; A Pilot Study for Efficacy Assessment. Retrieved from squarespace.com.

