Driven by a Vision
Dr. Dan Fischer has always been one of my mentors and one of the true freethinkers in dentistry. I have been in dentistry long enough to remember the “pre-Ultradent” period of dentistry, the period during which nothing was available in syringes. Dentistry got a lot more convenient when Dr. Fischer had the seemingly obvious (at least today) breakthrough to put top quality dental materials in easy-to-use intraoral syringes. My subsequent Ultradent breakthrough moment was when I used Astringedent® for the first time and could predictably stop bleeding prior to making an impression. Today, ViscoStat® and ViscoStat® Clear are two products I couldn’t practice without. And it is difficult to picture dentistry today without vital bleaching, and once again Dr. Fischer was the major impetus behind bringing this product to dental offices worldwide. In this issue’s Clinician Spotlight, Dr. Fischer shares how Ultradent rose through the ranks of dentistry to become one of the most respected businesses in the dental profession.
Dr. Michael DiTolla: Your company has significantly changed the way present day dentistry is delivered, especially intraorally with the introduction of your syringes. But if you had to pick one product that really exemplifies Ultradent, what would it be?
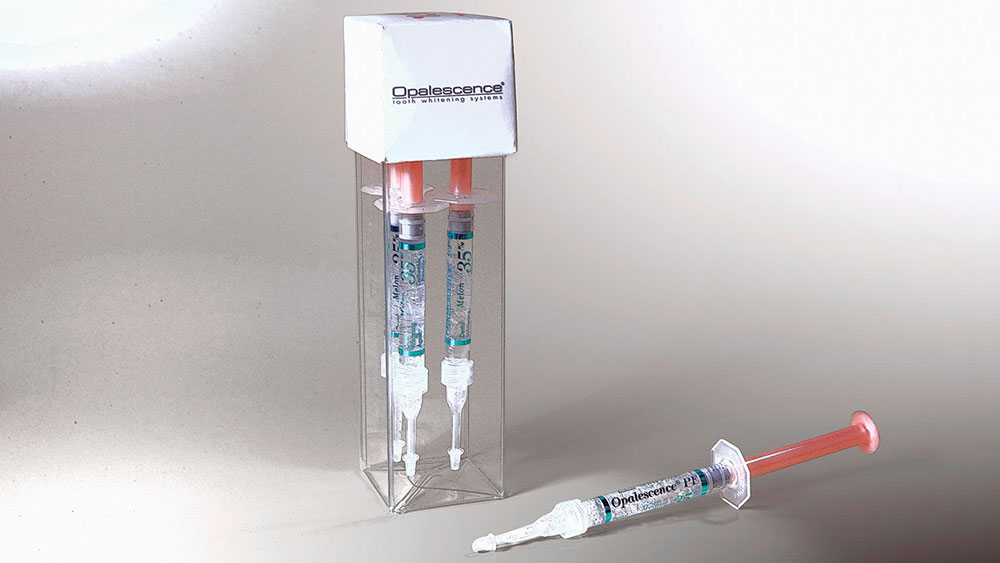
Dr. Dan Fischer: Of all the products Ultradent has invented and introduced, the most notable — which has touched the most human lives and brought advancement to dental care — is Opalescence®, the sticky, viscous bleaching gel delivered in a soft and thin scalloped tray. Opalescence, and similar products which followed its lead, brought a paradigm shift to dental care and esthetics. In-office bleaching procedures have made an impact on dental care. However, effective, predictable and comfortable at-home whitening agents have affected a much larger number of humans, particularly those who can’t afford in-office treatment. And even when we consider in-office bleaching separately, most patients attain their best whitening results via at-home systems with custom trays or the now disposable Opalescence Trèswhite™ Supreme system out-of-office procedure.
MD: I have always been amazed how patients start to care more about their dentition and the appearance of their smile after the bleaching of their teeth; it is an amazing transformation. If you had to pick one product related to restorative dentistry that defines Ultradent, which would you choose?
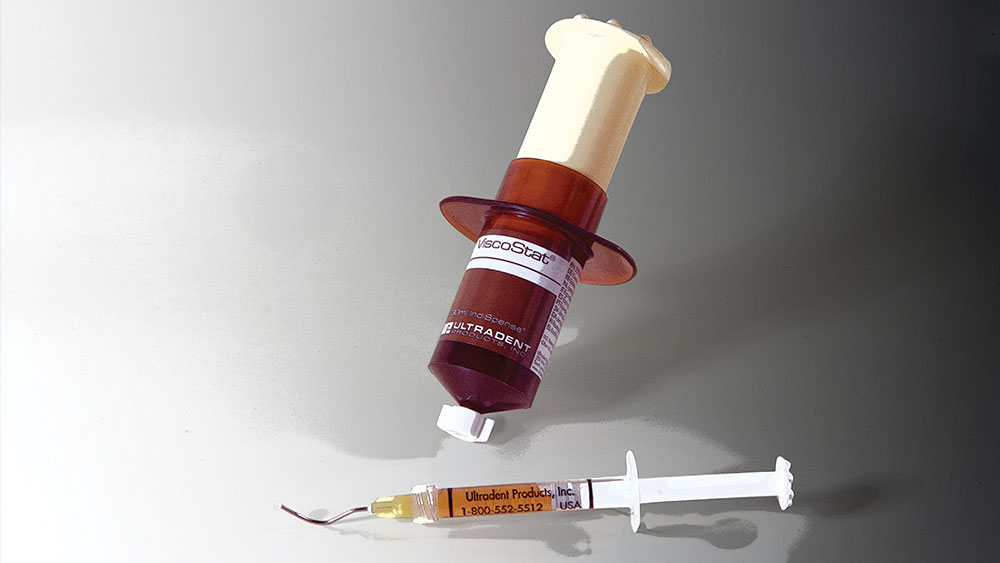
DF: With regards to operative dentistry, our tissue management products have helped to raise the quality of taking impressions with superior results for indirect restorations. Additionally, bonding with direct placed materials now occurs predictably with credible results when working subgingivally. In reality, there is nothing a dentist can do from a quality standpoint when bleeding is present. Astringedent (and later Viscostat and Viscostat Clear) and the Dento-Infusor® Tip brought rapid and predictable hemostasis for the very first time. In addition, the design of the Ultrapak® cord enabled easier and more rapid packing for tissue displacement.
MD: I completely agree that gingival inflammation and bleeding is a huge obstacle to ideal restorative dentistry. However, when backed into that corner and operating in a less than ideal field, Ultradent’s hemostatic products have saved me on numerous occasions! You now have roughly 25 years of clinical experience; tell me about the biggest change you have seen in our profession.
DF: The biggest change in dentistry centers on the patient, who now lives a longer, fuller life. Advancements in medical technology and lifestyle habits in recent decades have allowed humans, particularly those in the United States, to delay the clock of life by another 10 to 20 years. As a result, it’s become the dentists’ responsibility to see that patients keep their teeth for a prolonged period of time. Because of the need for our patients to stay dentate, our goals should focus on preservation of natural dentin, enamel and supporting tissues.
MD: So, it sounds like your philosophy embraces the least amount of dentistry necessary to achieve both yours and the patient’s goal. But not every professional practices this; it has always surprised me how many dentists are willing to sacrifice healthy enamel for the sake of esthetics. What are your thoughts on this?
DF: The patient now expects the ultimate level of esthetics. While there are some dental technologies that have kept pace, there are still some dentists who immediately opt for highly invasive and expensive procedures when less invasive and more affordable procedures may be a better choice. When I graduated as a young dentist we were always challenged by the dentists who wrongfully practiced “roundhouse” dentistry, crowning every tooth whether it was needed or not. Unfortunately, ethical dentists today find themselves challenged by those dentists who bring good practice sense and even the credibility of our profession into jeopardy when they choose procedures that are highly marketed or when applied considered “overtreatment.”
MD: Would you agree there are still a large number of conservative dentists who are not so willing to sacrifice enamel at the drop of a hat?
DF: Fortunately, there are many caring, ethical dentists in the profession. Our greatest opportunity is to become more serious in realizing that as patients live longer, we need to postpone more invasive procedures (should they be needed at all) as a final option. Also, we should keep in the front of our minds that each patient is our brother, sister or family member, lest we do no harm. I respect those who listen to the needs and values of the patient by keeping the three “E”s connected and in balance: Ethics, Esthetics and Economics. Dentistry is about people. We should never forget that our patients are people. Human principles and ethics don’t change. Only the tools and technologies for treating them do.
MD: Ultradent has also made some huge inroads in the field of direct placed restorations. What changes have you seen there?
DF: Obviously, the material of choice for the direct placed restoration (and 85% of the dentistry in the world is the direct placed restoration) has changed dramatically. Amalgam is moving away from the forefront, gold foil was gone some time ago, and bonded esthetic resin restorations are becoming the treatment option of choice for most clinicians. This is a positive change, but only if quality bonding agents and resin materials are used with methodical attention to detail. I believe that a quality bonded resin restoration is superior to a quality amalgam. However, a poor amalgam is better than a poor resin restoration. Thus, the emphasis in the material sciences has moved away from gold foil and amalgam to resins, including resins containing minority percentages of glass ionomer. Today, one seldom sees research for amalgam. When was the last time anyone found an amalgam advertisement in a dental journal? It is interesting to think about the reasons why this is the reality.
Quality adhesive dentistry and resin-based filling materials have completely changed tooth preparation design, particularly for the direct restoration. Instead of subtracting from the tooth to accommodate for the material, the clinician can excise principally the pathologic lesion (hopefully to mineral mother dentin and enamel) and then use an uncompromising bonding material. This is followed by materials that attach intimately with the bonding layer (namely resin-based composites), not only to restore anatomical needs but to restore the integrity of the tooth. I like to refer to this as “listening to the needs of the tooth” rather than choosing procedures that are dictated by the needs of the material.
MD: That is a great point, and I love your philosophy of letting the tooth choose the appropriate restorative material. It certainly benefits the patient when we focus on minimally invasive dentistry.
DF: I feel like a hypocrite if I discuss important concepts of minimal invasive dentistry without the mention of preventive dentistry, which I believe to be the single most important part of minimal invasive dentistry today. This includes fluoride varnish, a treatment that has the potential to prevent the destructive caries process in a treatable high-risk patient, starting at one year of age. This is a significant change in the current standard of care, so there needs to be a great deal of education (which has yet to occur). Research and work from associations such as AAPD and ADA have done a great deal to promote the subject since more patients will be requiring preventive treatments, such as fluoride.
MD: The last time we had lunch you really opened my eyes as you spoke about your quest to cure caries. I haven’t really heard anyone in commercial dentistry talk about this, and with such passion. Can you tell me a little more about your ambitious goal?
DF: Caries is an international epidemic that cannot be neglected, and Ultradent feels empowered to find a cure for this deadly disease. Thankfully, we’re progressing with our goals in sight. Unfortunately, however, Second and Third World countries have adapted to the Western worlds offerings of high-sugar foods and other poor dietary habits, while proper dentistry cannot be afforded and has yet to be applied. This is a serious humanitarian issue. It’s shameful that so many people don’t get treatment until after this infectious disease has already done its damage. We have taken this course with so many other diseases. Even if we bring a cure to caries today, we’d still need more dentists. That’s because people are keeping their teeth as they age. The average lifespan is now 77 years old, a number that slowly continues to increase every year. And with the soon to be aged baby boomers arriving to their latter years, the true challenges lay ahead. We should consider the 80 million+ Americans reaching their older years, moving to assisted living and nursing care centers with their teeth still in place. Think of all the salivary glands drying up, the rampant resultant root caries, not to mention periodontal disease. Additionally, Americans of all ages will want and demand a higher level of esthetics. If we don’t get serious about preventing caries today, I don’t know how we’ll meet our obligation to our fellow humans.
MD: It sounds as though the curing of caries may be the biggest medical breakthrough in the history of mankind, and it would be humbling to witness dentistry play a leading role in that accomplishment. Moving on to another note, vital bleaching is something most of us take for granted. What was bleaching like when you graduated from dental school?
DF: Wow, the world of whitening is vastly different now compared to when I was a young dentist. We learned how to do in-office whitening with heating irons or lamps invented in the early 1900s, but take-home whitening is the principle technology that resulted in the monumental paradigm shift. Henry Ford stated, “True progress is not realized until technology is made available to masses.” In this sense, take-home bleaching brought “true progress.”
More recently, we’ve learned the benefits of including small quantities of fluoride and potassium nitrate in bleaching compositions. Our Opalescence PF product is formulated as such. Scientific evidence demonstrates that low doses of these chemistries delivered over significant treatment times — as is afforded with tooth bleaching, be it for 30 minutes a day or overnight — facilitates significant hardening of enamel, complete with resultant reduction in susceptibility to caries and reduction in tooth sensitivity. The advertising claim of zero sensitivity is misleading. It’s a marketing gimmick, not a scientific fact. Fluoride and potassium nitrate are scientifically proven to help in several ways, including a significant reduction in sensitivity for bleaching procedures. Fortunately, saliva contains an abundance of calcium, so the addition of calcium in bleaching formulas has not proven to bring value.
The increase in esthetic awareness and procedures, such as tooth whitening, has also had the added benefit of bringing patients into the dental office. These patients might not have scheduled appointments otherwise. By having them in the dental chair, we are able to reach out, educate and treat them for other oral health issues. If someone were to ask me what the most rewarding part of being a dentist or manufacturing dental products is, I would say that we have the rare opportunity to change people’s lives for the better.
MD: Tell me a little about the Ultradent corporate culture. When I have visited your facilities in the past, it has always felt like Ultradent does things a little differently than other dental companies.
DF: Unlike other companies in the industry, Ultradent is really three companies wrapped into one — a strong R&D, manufacturing and sales/education vehicle. We’ve taken advantage of our strengths in these areas. In addition to manufacturing our own syringes, delivery tips and chemical formulations, we also manufacture most of our secondary packaging. In fact, we manufacture more than 95% of what we sell. Our employees eat, drink and sleep the realities of changing technologies and the values they bring at every front. We also count our blessings every day, as we’ve learned that not doing so erodes what we’ve become best known for — namely a company that is both progressive and trustworthy. Yes, we also make mistakes, but we’re committed to quickly addressing and then learning from them when they do occur. Our people, no matter what department they reside in, revel in this reality. Ultradent has some of the best talent in the industry under its roof — 800 colleagues and counting, a team we champion as the Ultradent family. In a fun way, our top customers proudly embrace and tout their membership in the Ultradent family, too. We are truly fortunate and realize a large part of it is due to living up to our value of hard work. The harder we work, the luckier we are.
We operate with a very specific vision at Ultradent, and that is this: we are passionately driven to enrich quality of life by improving human oral healthcare throughout the world. Each product we consider, manufacture or sell must meet the objectives of our vision in a direct way, or as income that helps us move closer to our vision. Every aspect from R&D onward is geared to embrace new technologies, with the main goal of supporting minimal invasive dentistry.
MD: I look at Ultradent now and it all makes perfect sense. But take us back to your first days as a dentist, when you began to see patients after graduating from dental school. How did the seeds of Ultradent get planted in your head?
DF: In the later part of the ’70s, I was driven to find better solutions for controlling bleeding. My passion in the early days was full-mouth reconstruction, making quality impressions. This is the most important step for the indirect procedure and, as we all know, the fulcrum to success or failure hinges on the clinician’s ability to obtain quality hemostasis complete with tissue displacement. Driven by this passion, I set up a lab in the basement of my home. Upon completion of each day in my dental practice, I’d go to the lab, draw blood on myself and work with different chemistries. From this continuous experimentation resulted the coagulative, hemostatic agent Astringedent and the Dento-Infusor, a unique delivery tip attached to a syringe. Together, these inventions enabled predictable profound hemostasis for the first time.
MD: I am fascinated by the thought of you drawing your own blood to develop these products — that’s dedication! And once you had Astringedent working, how did you know it was time to start the company?
DF: My initial intent was not to start a company. I introduced Astringedent and the Dento-Infusor Tip to a number of large companies, which said either they couldn’t see the value in the products or they weren’t willing to pay for them. At this point, I was determined that if this technology were to come to dentistry we’d need to work as family to make it happen. The first product, Astringedent, was literally made on my kitchen table.
The development of the in-house Astringedent system is an interesting story. We cut part of a five-gallon water cooler bottle for the base. Metal pipes were welded to the cooler base for the legs. After meticulous sanding and surface preparation, the components were painted with white epoxy paint. Using a two-hole laboratory neoprene cork, two one-fourth inch glass tubes were added. The first tube was inserted in the bottom of the five-gallon bottle so that when the bottle of Astringedent was inverted, the cooler top prevented a vacuum effect as the contents of the bottle were slowly dispensed. The other tube was inserted inside the orifice of the jug. A surgical hose was attached to the system with a three-inch length of tubing for reaching into small 30cc bottles for filling when a hose clamp was depressed. Each bottle was filled by older children with sanitized hands wearing their caps and gowns. I made a jig that enabled them to wrap pressure adhesive labels directly onto the filled bottles. Although the system was clean and functional, these devices didn’t exactly meet FDA specifications and requirements.
MD: It must have been fun for your kids to participate in the family business on such a hands-on level. How did you eventually meet those FDA guidelines?
DF: Our greatest challenge was becoming FDA compliant, and this was a project that took four years to achieve. It was an incredible journey and learning experience that resulted in long, colorful stories that would exceed the parameters of this article. First, we needed to get the product compliant. Secondly, we needed our manufacturing processes to become compliant. Eventually — and after a lot of blood, sweat and tears — we satisfied the needs of FDA, complete with an appropriate facility, GMPs, etc. Ultradent soon became one of the first dental companies to manufacture syringe-packaged and delivered materials. On January 1, 1995, Ultradent became the first dental company in the world to receive CE mark for Europe, beating even the European dental manufacturers in this important quest.
MD: So, after you invented the product and were granted FDA approval, I suppose the only thing left to do was find a target market and sell it!
DF: After meeting the requirements of the FDA, we wanted to address the marketing and sales of our products. We quickly came to realize that invention was the “easy” part. Educating customers about our products and encouraging them to purchase and use them was the most difficult and expensive part of the process. Paying for other costly marketing activities was also extremely strenuous. For some time, our exhibits at tradeshows, advertisements and similar activity was purchased with income from my dental practice.
MD: Many companies decide to sell through dealers early on, but Ultradent has always had their foot firmly planted in the direct sales market. How was that decision reached?
DF: We learned that in order to educate and sell new technologies we couldn’t have a third party selling to customers, as they often didn’t fully understand our products. We were teaching dentists to rub firmly areas that were bleeding in order to stop the bleeding! Human nature, intuition and practical training rebelled against such thoughts for understandable reasons. Because of this, we realized the need to be as close to our customers as possible to help them succeed. This way they could also obtain, for the first time, profound hemostasis to bring predictability and quality to their impression making and other procedures. We continue to learn the importance of dealing directly with our dentist customers, maintaining as close a relationship as possible so that we too can learn from them. “Listen to your marketplace; listen to the needs of those you serve”; “The greatest of all is the servant of all.” Marketing and sales continues to be one of the most expensive and important requirements of our operation.
Ultradent grew rapidly in the ’80s and ’90s.The company currently manufactures and packages more than 500 materials, devices and instruments. We are now a global company with a drive to enrich the quality of life and improve human oral care throughout the world, and have in place a fantastic network of distributors in more than 100 countries with our own subsidiaries in Germany, Italy, Brazil and Japan. Ultradent also sponsors continuing education seminars and training for dental students, practicing dentists, prominent dentists and lecturers from around the world. We often invite these groups to our facility to provide training on the company’s latest products and techniques. To stay engaged with these professionals, it is important that I still maintain a part-time practice. I don’t believe Ultradent would be as successful, or that I would truly enjoy the success as much, if not for the opportunity to connect with my patients.
MD: I completely agree with you. If you stop practicing it would be way too easy to forget how the bad days in dentistry feel. And it’s those tough days that ignite your inspiration to create products that make those days more tolerable. How long did Ultradent remain a family business?
DF: In the early days of our operation, manufacturing was one of our more predictable undertakings (albeit very demanding with many long hours). With 14 children, we even had the human resources necessary, but it still required many late night sessions. Including my children in this effort had the side benefit of helping them grow into adulthood with a strong work ethic in place. Addressing distribution and administration issues could have been handled readily with a set of unique new products in place if we’d been an established company. But we were a struggling, young family business trying to make it on a shoestring budget. In fact, we mortgaged everything we owned and personally guaranteed every loan for many years. When money became very tight, we’d withdraw money against our personal credit cards. We maintained these cards at the highest limit possible.
MD: Wow! To say you were personally invested in this business is the absolute truth. It’s inspiring to hear your story and to know the risks you took to bring this company to fruition. Did you also struggle with the technological aspects of putting this business together?
DF: Technology came to us in time, albeit sometimes at a challenging pace. At times it stopped us in our tracks. Our first computer was an Ohio Scientific 8-inch floppy disk, which was more like a glorified word processor. This came complete with a tractor feed printer, which required us to pull outward continuously with moderate pressure as it printed or it would slip a cog and jam. We couldn’t afford the standard at the time, the mainframe computer. Instead, we pushed the limits with each and every new version of the existing PC, driving it to handle work beyond its designated capabilities. We finally reached the capacity of a high peak hard drive of 10 megabytes, moving up to a 286, after being destroyed by a tape backup system that shredded rather than recorded.
Because we had difficulty finding a knowledgeable and experienced PC guru to help us through the challenges, we put our oldest son (at age 15) to the task. Erwin’s experience: the Atari 800 computer. His passion: make it happen, and through the night if necessary! We had crashes, we had tears, we had jubilation, we had mistakes, we had victories, but it was all we. And we succeeded. I prompted Erwin to continue with college but he made the decision to drop out in his junior year to continue working, thus foregoing a college education in the process. Erwin and I both believe that he received a greater computer education than what universities at the time could have provided, but he did make a sacrifice. (Today, he continues to work nights for his college degree and principally for the learning experience.) The results have been a reward to all of us. It is seldom that a company our size is so fully integrated on a software program as powerful as Oracle. Oracle is seldom incorporated until a company is at least five times our size. How is this possible? Erwin had a commitment and a passion to push it through. He didn’t do it alone, however. He worked, and continues to work, with a team of some 30 top notch IT guys, a powerful “can do” team that has grown significantly. This fabulous team continues to push us to higher spheres each day, each year.
MD: You are going to prompt all the dentists who want to start a business to put their kids to work! Can you share with us a little about the current goals of Ultradent?
DF: First and foremost, we must continue to focus on listening to the needs of the marketplace, particularly the needs we have a skill at addressing. Second, we must never become complacent. Third, we must continually be driven to grow. And finally, we must always strive to learn and adapt to new technologies in our R&D and manufacturing. For example, we’ve been told that we have more robotics than any company outside of the automobile industry on the west side of the Mississippi. We believe that for the American industry, in this case the dental industry, to maintain a competitive edge over emerging giants (such as China and India), we must outperform others in R&D and automate as much as we reasonably can.
For more information about Ultradent Products, call 888-230-1420 or visit ultradent.com.
Astringedent, ViscoStat, Viscostat Clear, Opalescence and Ultrapak are registered trademarks of Ultradent Products, Inc. (South Jordan, Utah). Opalescence Trèswhite is a trademark of Ultradent Products, Inc.