- PDAC (Pricing, Data Analysis and Coding) contractor-verified for Medicare reimbursement: Code E0486
- Advances the mandible 0.25 mm with each 180-degree turn of the key
- Stronger than surgical stainless steel, the hardware is made from nickel-free cobalt-chromium
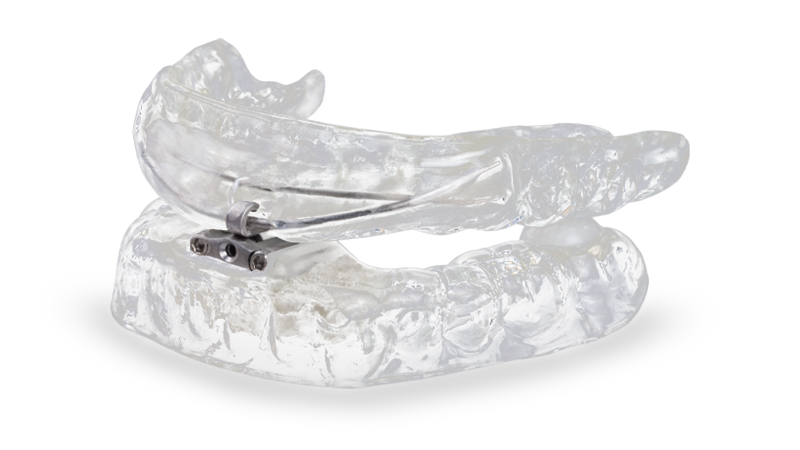
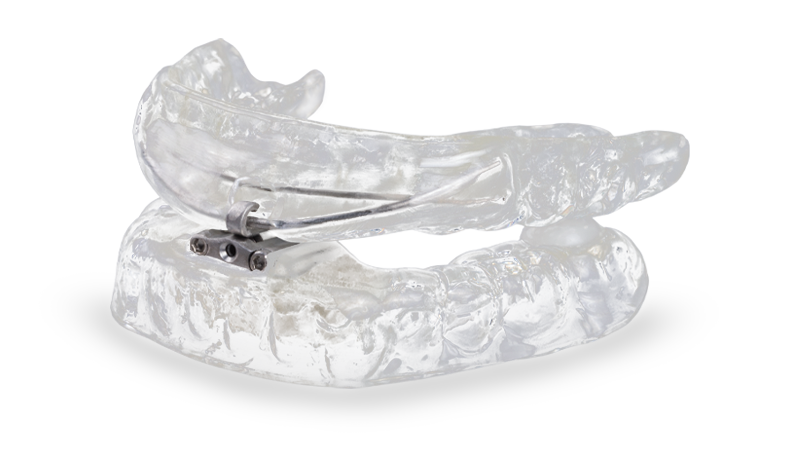
dreamTAP™
*Price is per appliance and does not include shipping or applicable taxes.
Developed with advanced technology, the dreamTAP™ appliance is designed to treat snoring and obstructive sleep apnea.
Embrace Life with Better Sleep
The dreamTAP is among the TAP® family of appliances for the treatment of snoring and obstructive sleep apnea (OSA). The Thornton Adjustable Positioner (TAP) is based on the same principle as cardiopulmonary resuscitation (CPR) and is designed to keep the airway open to allow air to pass. A constricted or collapsed airway causes snoring and OSA.
Developed with advanced dental technology, the dreamTAP appliance reduces sleep apnea-associated health risks without the need for surgery, medications or other more cumbersome therapy. The dreamTAP holds the lower jaw in a forward position, maintaining a clear airway to reduce snoring and improve breathing. The dreamTAP features strong, biocompatible cobalt-chrome alloy (Co-Cr) hardware and has three hook sizes for increased range of adjustment. An adjustment key enables the patient to modify the lower jaw’s protrusion until a comfortable, effective position is achieved.
TAP is a registered trademark of Airway Management Inc. dreamTAP is a trademark of Airway Management Inc.
Patient Satisfaction
Oral appliance therapy (OAT) is very effective in treating patients with sleep-disordered breathing, with a compliance rate shown to be as high as 90% over a 2.5-year period.1
OAT is associated with greater patient satisfaction than nasal continuous positive airway pressure (CPAP) therapy.2
In a randomized crossover trial comparing OAT to CPAP in patients with obstructive sleep apnea, about 81% preferred OAT.3

Improved Health
Among patients with obstructive sleep apnea, both continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) were associated with reductions in blood pressure. Network meta-analysis did not identify a statistically significant difference between the blood pressure outcomes associated with these therapies.4
A mandibular advancement device for obstructive sleep apnea reduces nocturnal blood pressure in women.5

Prevalence of Snoring and Obstructive Sleep Apnea
About half of the U.S. adult population experiences some form of sleep-disordered breathing.
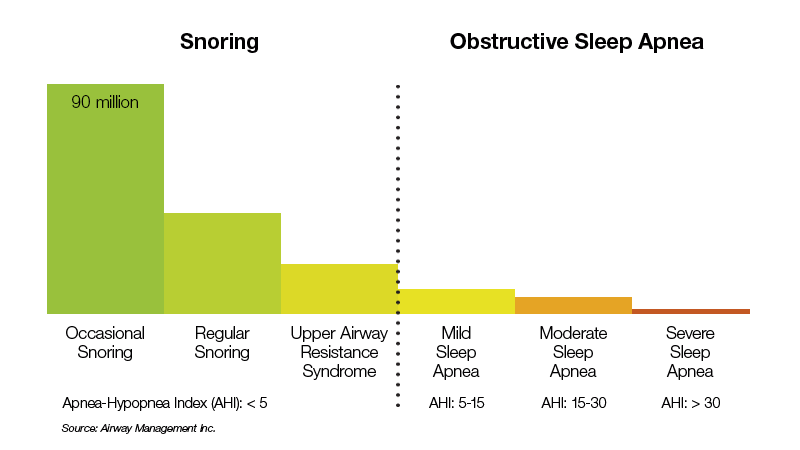
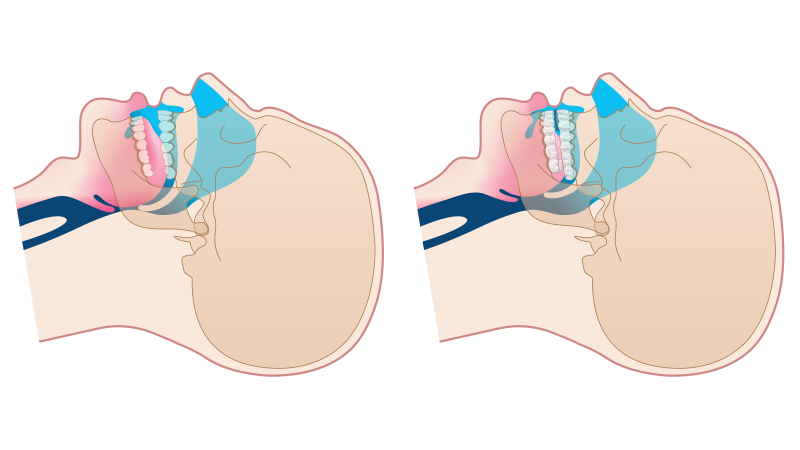
Treating Patients with the dreamTAP
The dreamTAP is designed to keep the airway open to allow air to pass during sleep.
I used to wake up at night gasping for air and snoring … Now that I have my appliance, I sleep better and longer, and no longer feel sleepy throughout the day. My wife is impressed with how my appliance has helped our day-to-day lives.
Indications
The dreamTAP™ is intended to reduce or alleviate nighttime snoring and sleep-related breathing disorders, including obstructive sleep apnea (OSA).
Material Composition
Thermoformed material and cobalt-chrome alloy hardware
In-Lab Working Times
5 days
Available Colors
Clear
Pricing
|
dreamTAP™(PDAC-approved) (buy 1)
|
$528.001 |
|
Glidewell Clinical Twinpak(buy 2)2
|
$1,028.001 |
Digital file storage is available for this product. See Scan & Save Services.
Glidewell Clinical Twinpak is valid for two appliances for the same case.
Pricing is subject to change and does not include shipping or applicable taxes.
Policies & Warranty
NO-FAULT REMAKE POLICY: Glidewell is pleased to process all remakes or adjustments at no additional charge if requested within the warranty period and accompanied by the return of the original appliance.
LIMITED WARRANTY/LIMITATION OF LIABILITY. Glidewell (“the lab”) warrants that all dental devices (a “device”) are made according to your specification and approval in the belief that the device will be useful and MAKES NO OTHER WARRANTIES INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. Subject to the return of a device that is placed and then fails, the lab will repair or replace the device without charge for the cost of materials and workmanship or refund the original price paid, at the lab’s option, for up to 2 years for the dreamTAP appliance.
Procedures
References
1. ^ Yoshida K. Effects of a mandibular advancement device for the treatment of sleep apnea syndrome and snoring on respiratory function and sleep quality. Cranio. 2000 Apr;18(2):98-105.
2. ^ Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA. A randomized crossover study of an oral appliance vs. nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996 May;109(5):1269-75.
3. ^ Tan YK, L’Estrange PR, Luo YM, Smith C, Grant HR, Simonds AK, Spiro SG, Battagel JM. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Eur J Orthod. 2002 Jun;24(3):239-49.
4. ^ Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs. mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015 Dec 1;314(21):2280-93.
5. ^ Rietz H, Franklin KA, Carlberg B, Sahlin C, Marklund M. Nocturnal blood pressure is reduced by a mandibular advancement device for sleep apnea in women: findings from secondary analyses of a randomized trial. J Am Heart Assoc. 2018 Jun 21;7(13).

