- PDAC (Pricing, Data Analysis and Coding) contractor-verified for Medicare reimbursement: Code E0486
- Metal-free with 14 mm range of patient adjustability
- Each case comes with a Mouth Shield nasal breathing trainer and an AM Aligner®
- No bite registration required

flexTAP®
*Price is per appliance and does not include shipping or applicable taxes.
Helping to provide a restful night’s sleep, the flexTAP® is a premium mandibular advancement device (MAD) for the treatment of snoring and obstructive sleep apnea.
flexTAP — That Was Easy
Provided as a complete airway management system, the flexTAP appliance defends against partial or complete collapse of the upper airway, encourages nasal breathing and manages tooth movement, a known side effect of mandibular advancement therapy. Comprised of three components, the system includes:
- flexTAP MAD — This appliance engages the patient’s upper and lower dentition. The micro-adjustment of the mandible results in tension of the muscles and ligaments of the upper airway to maintain a patent airway. The design allows for more airway and tongue space with less protrusion than other TAP appliances.
- Mouth Shield — Designed to promote nasal breathing, this nasal breathing trainer reduces the drying effect and excessive saliva that is common with oral breathing.
- AM Aligner — This device records the patient’s natural pretreatment bite. The AM Aligner is worn each morning after treatment with the flexTAP appliance to exercise the jaw back into normal occlusion should any change have occurred while using the MAD overnight.
As a line extension to the TAP family of products, the flexTAP appliance delivers a best-in-class clinical experience.
flexTAP and AM Aligner are registered trademarks of Airway Technologies, LLC. Mouth Shield is a product of Airway Management Inc.

Patient Satisfaction
Oral appliance therapy (OAT) is very effective in treating patients with sleep-disordered breathing, with a compliance rate shown to be as high as 90% over a 2.5-year period.1
OAT is associated with greater patient satisfaction than nasal continuous positive airway pressure (CPAP) therapy.2
In a randomized crossover trial comparing OAT to CPAP in patients with obstructive sleep apnea, about 81% preferred OAT.3

Improved Health
Among patients with obstructive sleep apnea, both continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) were associated with reductions in blood pressure. Network meta-analysis did not identify a statistically significant difference between the blood pressure outcomes associated with these therapies.4
A mandibular advancement device for obstructive sleep apnea reduces nocturnal blood pressure in women.5

Prevalence of Snoring and Obstructive Sleep Apnea
About half of the U.S. adult population experiences some form of sleep-disordered breathing.
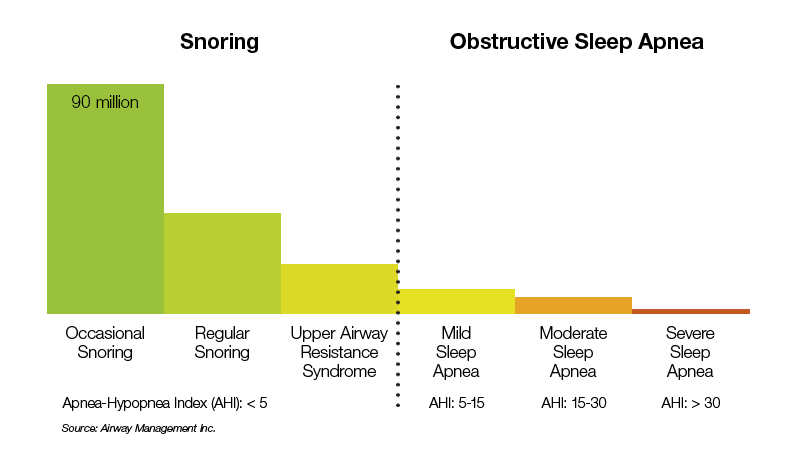
I’m helping patients to get happier and healthier with the flexTAP. When everybody’s happy and healthy, that contributes to the growth of my business. It’s a win-win situation.
Indications
The flexTAP is intended to reduce or alleviate nighttime snoring and obstructive sleep apnea (OSA).
Material Composition
Biocompatible, irradiated thermoplastic and silicone
In-Lab Working Times
5 days
Available Colors
Blue
Pricing
- flexTAP sleep apnea device: $395* per appliance
- flexTAP sleep apnea device (Glidewell Clinical Twinpak™): $765* for 2 appliances
*Digital file storage is available for this product. For more information on our Scan & Save services, call us at 800-407-3326.
Pricing is subject to change and does not include shipping or applicable taxes.
Policies & Warranty
NO-FAULT REMAKE POLICY: Glidewell is pleased to process all remakes or adjustments at no additional charge if requested within the warranty period and accompanied by the return of the original appliance.
LIMITED WARRANTY/LIMITATION OF LIABILITY. Glidewell (“the lab”) warrants that all dental devices (a “device”) are made according to your specification and approval in the belief that the device will be useful and MAKES NO OTHER WARRANTIES INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. Subject to the return of a device that is placed and then fails, the lab will repair or replace the device without charge for the cost of materials and workmanship or refund the original price paid, at the lab’s option, for up to 3 years for the flexTAP appliance.
References
- ^ Yoshida K. Effects of a mandibular advancement device for the treatment of sleep apnea syndrome and snoring on respiratory function and sleep quality. Cranio. 2000 Apr;18(2):98-105.
- ^ Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA. A randomized crossover study of an oral appliance vs. nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996 May;109(5):1269-75.
- ^ Tan YK, L’Estrange PR, Luo YM, Smith C, Grant HR, Simonds AK, Spiro SG, Battagel JM. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Eur J Orthod. 2002 Jun;24(3):239-49.
- ^ Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs. mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015 Dec 1;314(21):2280-93.
- ^ Rietz H, Franklin KA, Carlberg B, Sahlin C, Marklund M. Nocturnal blood pressure is reduced by a mandibular advancement device for sleep apnea in women: findings from secondary analyses of a randomized trial. J Am Heart Assoc. 2018 Jun 21;7(13).

