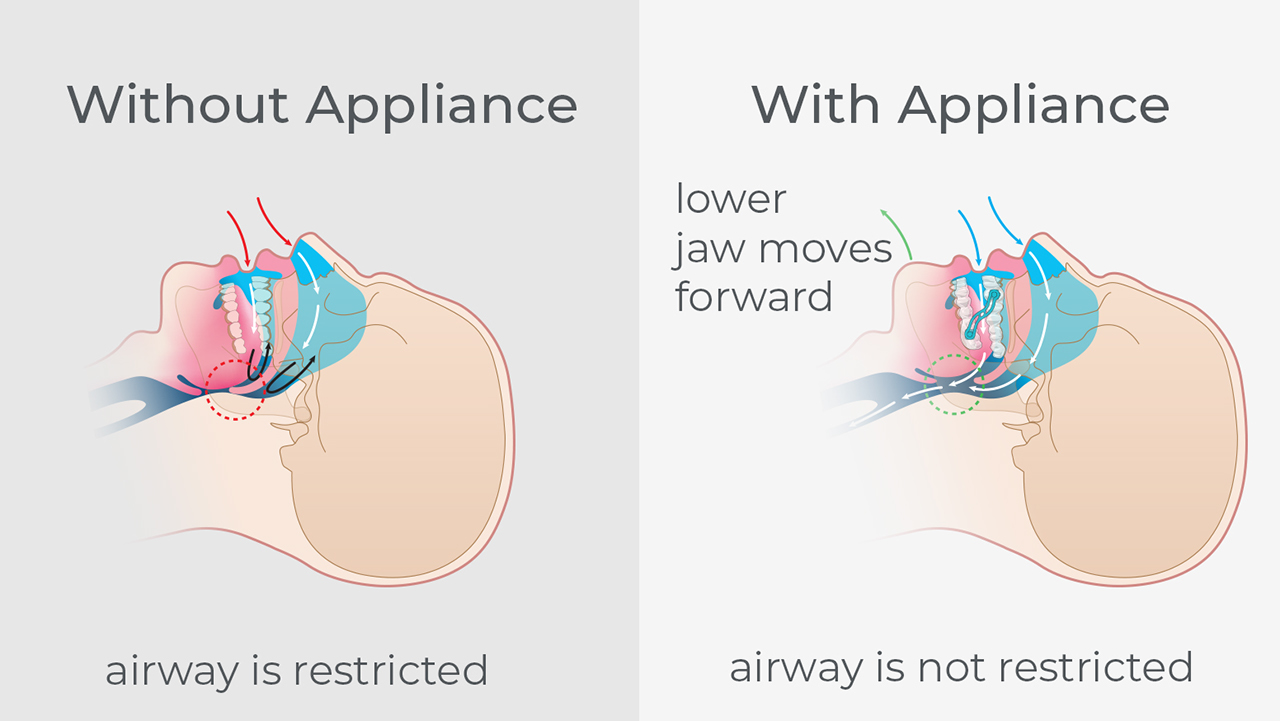
Snoring is due to a partially collapsed airway. As tissues of the airway relax during sleep, the airway partially collapses, restricting airflow and causing vibration in the tissues, making the snoring sound. When the airway fully collapses, airflow is cut off and OSA occurs. Patients with untreated OSA may have an increased risk of developing cardiovascular disease, including difficult-to-control blood pressure, coronary artery disease, congestive heart failure, arrhythmias and stroke. Research also shows that OSA is associated with metabolic dysregulation, which affects glucose control and diabetes risk. It is important to note that there is a direct connection between snoring and sleep apnea: Although not every snorer has sleep apnea, every obstructive sleep apnea patient snores.
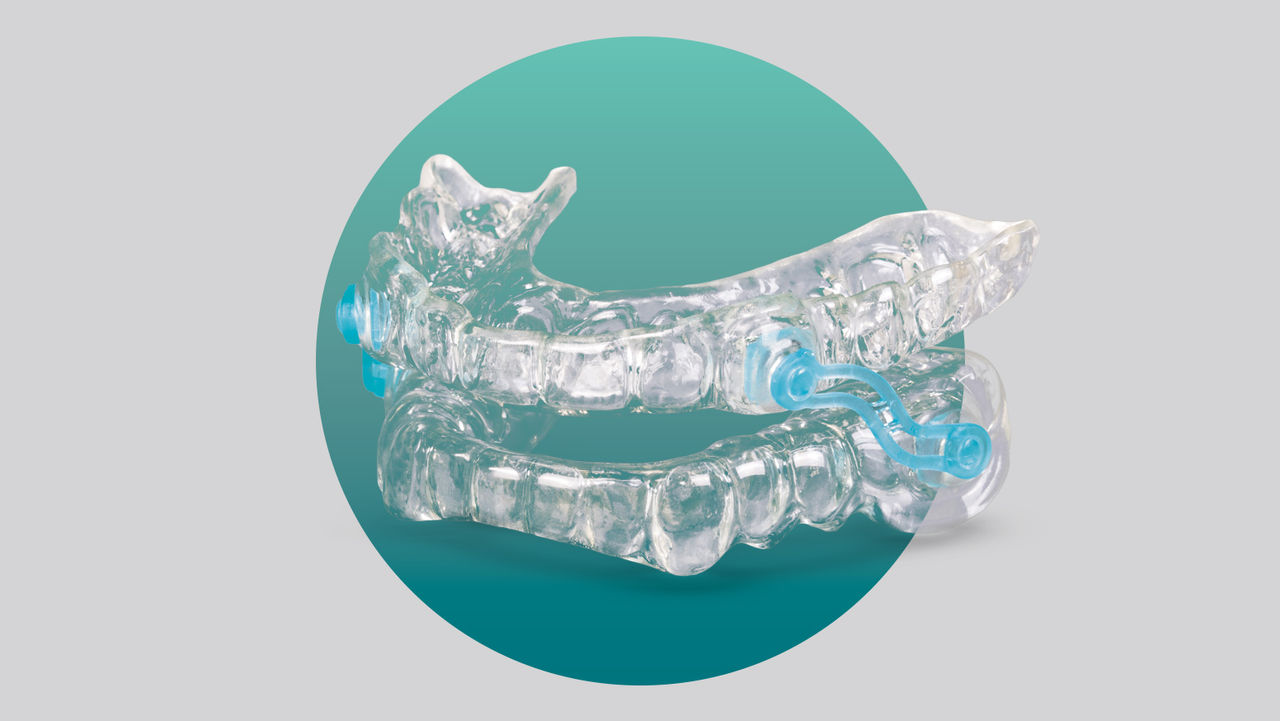
Mandibular advancement devices (MADs) have been clinically shown to be an excellent treatment for partial airway collapse. They look like an upper and lower mouthguard and work by engaging the teeth and holding the lower jaw forward, thereby maintaining an open airway. These mouthguards reduce upper airway collapse and eliminate vibration and the snoring sound. Many studies have shown that this protrusive jaw position is also a good way to reduce the number of OSA events per night, leading to a much deeper and restful night’s sleep.
Over-the-counter (available without prescription both in-store and online) snoring mouthguards are relatively inexpensive. The best examples of these devices form to the teeth by users placing them in boiling water for a few minutes, and then inserting them into the mouth and biting down for a semi-custom fit. This is a critical step to improved fit and function of the mouthguard. When it comes to sleep apnea, the FDA regulates all treatments for medical conditions. Sleep apnea is a medical condition, so a prescription is required to purchase treatment.
The key to using over-the-counter sleep devices is to assess the following questions: Do they work? Will they stop the snoring sound? Will they lower the apnea-hypopnea index (AHI), which is the key metric in measuring OSA severity? How do they compare to dentist-prescribed, fully custom laboratory-fabricated devices in the key areas of comfort, efficacy and compliance, which are very important for long-term use? Are these mouthguards clinically effective?
I have had many conversations over the years where it has been suggested that inexpensive, semi-custom, patient-made mouthguards may be a predictor of success. In fact, Kaiser Permanente® hospitals use the ApneaRx® mouthguard with a home sleep test to predict if the device is tolerated and effective before prescribing a fully custom, lab-fabricated oral appliance that is fitted by a dentist.
The ApneaRx is a semi-custom mouthguard. It is a micro-adjustable, thermoplastic (heat-molded) monobloc device that fixes the jaw in a set position. The patient forms the device in the same way as a traditional sports mouthguard. The thermoplastic material is placed in boiling water until it is soft enough to retain the impression of the teeth. The patient bites into the material and allows the material to cool and retain the shape of the dentition.