REFERENCES
- 2016 Frost & Sullivan survey of 506 U.S. residents treating their OSA.
800-854-7256 USA
Consider neck size as a risk factor for sleep-disordered breathing.

Snoring occurs because of a partially collapsed airway. The air rushing through the partially collapsed airway causes tissues to vibrate, creating the snore sound. Obstructive sleep apnea (OSA) makes no sound at all, until the increased effort to breathe awakens the sufferer with a gasp of breath. Once the person falls back to sleep, more snoring occurs until the throat muscles relax, allowing the fat tissues of the throat and tongue to fall back in the airway and seal the upper airway into another obstructed silence.
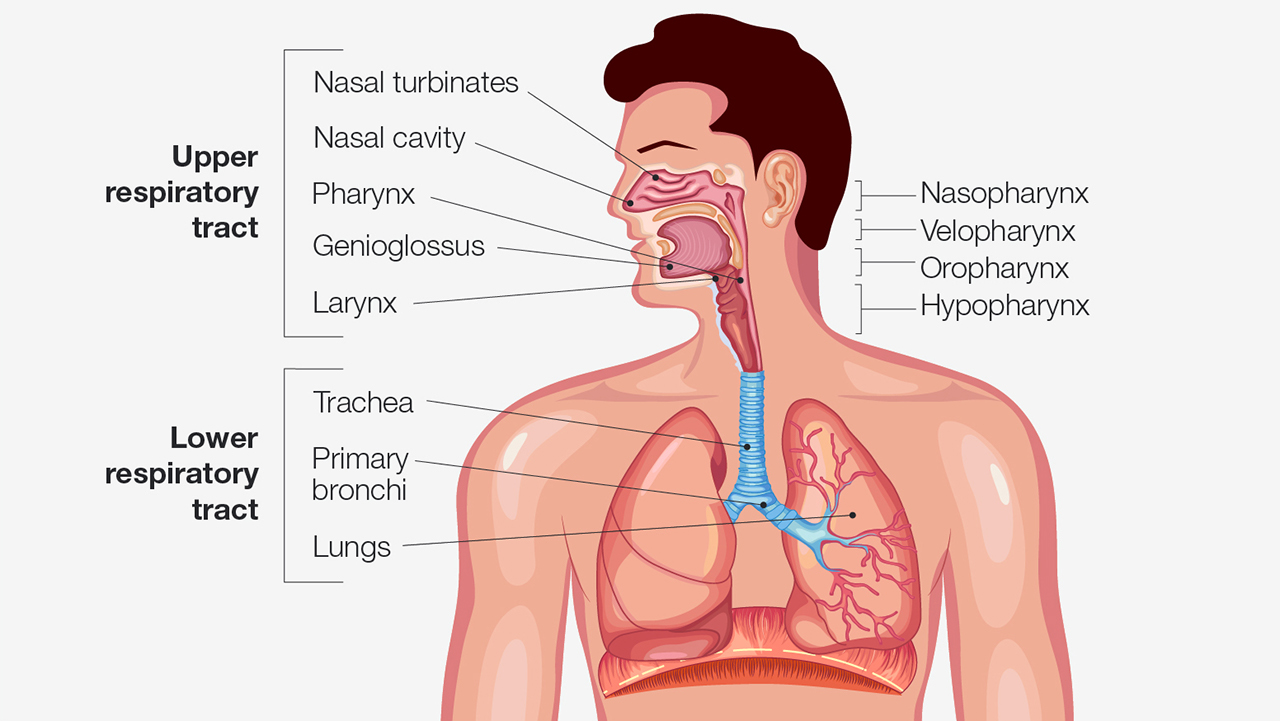
Upper and lower respiratory tract.
Snoring is a leading indicator of OSA, a condition that is characterized by airflow cessation, oxygen desaturation and sleep disruption. Comorbid conditions of OSA include heart disease, stroke, metabolic syndrome and type 2 diabetes, asthma, chronic obstructive pulmonary disease (COPD), and cancer.
Not every snorer has sleep apnea, but every OSA patient snores. The diagnostic link between neck size as an indication of risk for obstructive sleep apnea is well established and a key question on the STOP-Bang questionnaire. The STOP-Bang questionnaire is an easy, effective and reliable tool in predicting risk of OSA.
There are several factors that, when taken together with neck circumference, have been shown to predict risk of sleep apnea with reproducible accuracy:
During his lectures, dental sleep medicine pioneer Dr. L.W. Halstrom would frequently say, “Shirts with a neck size of 17 inches or larger for men and 16 inches or larger for women should come with a warning label.” The quote was usually shared just after lunch when the audience was a little sleepy and needed a dramatic pick-me-up.
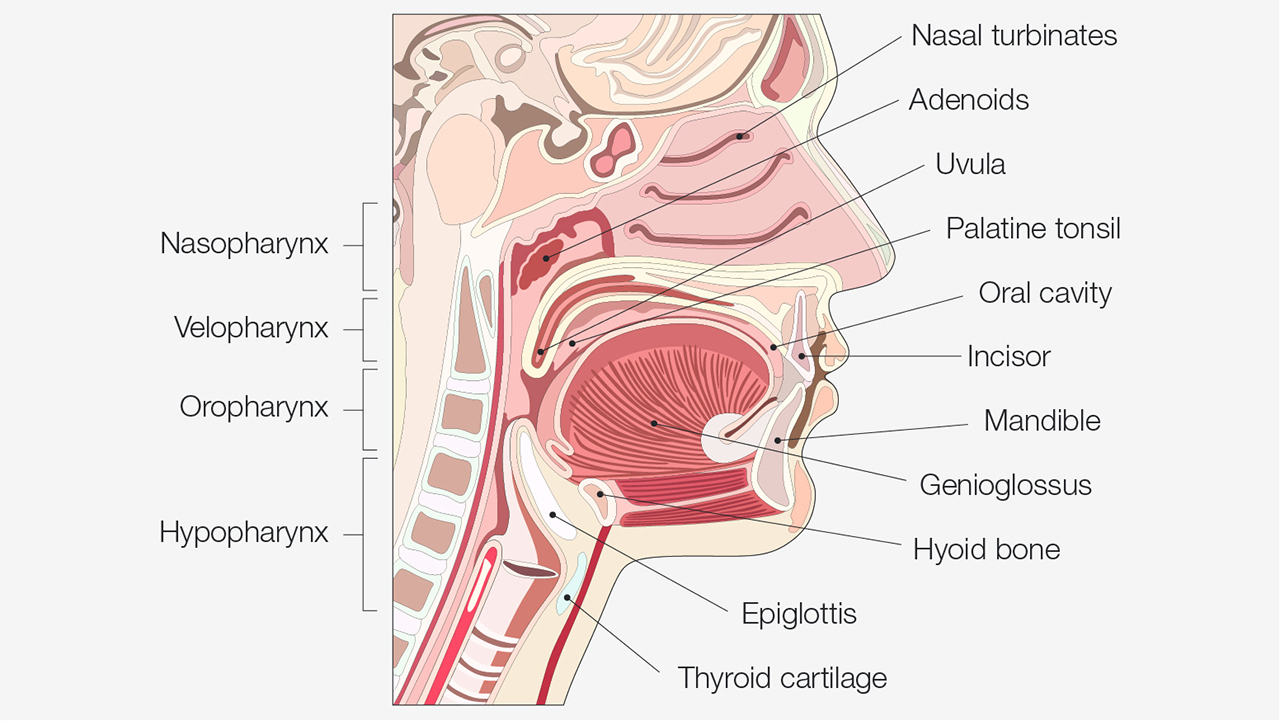
It is imperative to understand how neck size and body mass may work with existing anatomical features to create conditions in the upper airway that could lead to partial airway collapse or complete airway obstruction. The soft and bony structures of the pharynx are important in determining the size, structure and patency of the airway. The soft tissues include the tongue, soft palate, uvula, tonsils and pharyngeal walls. Bony structures are the mandible and the hyoid bone.
Upper respiratory tract.
A study published in the American Journal of Respiratory and Critical Care Medicine examined the contribution to obstructive sleep apnea of each of these anatomic structures. It was found that the narrowing of the pharyngeal walls is the primary cause of OSA, followed by tonsil enlargement, uvula enlargement and tongue enlargement, respectively.
In 2016, Frost & Sullivan surveyed 506 U.S. residents who sought treatment for their OSA and found that 70% sought treatment due to bed partner disturbance and 56% sought treatment due to excessive daytime sleepiness.1 When a patient presents in the dental office with a primary complaint of simple snoring, the first step in the process is to have the patient complete a STOP-Bang questionnaire. This will allow the clinician to identify the patient’s OSA risk. It is critical for the clinician to remember that not all snoring is sleep apnea. A straightforward questionnaire coupled with simple measurements of neck circumference and a calculation of body mass index (BMI) will go a long way to estimate risk of sleep apnea.
If sleep apnea is suspected, initiation of provisional mandibular advancement device (PMAD) therapy is indicated. When the patient has a primary complaint of snoring, that can be treated easily and reversibly with a mandibular advancement device like the Silent Nite® Sleep Appliance or EMA® (elastic mandibular advancement) oral appliance. Provisional therapy is the temporary dental treatment of sleep-related symptoms until a physician’s diagnosis is obtained. It is important when treating a patient in this protocol to obtain informed consent from the patient and provide a referral to the patient’s primary care physician or a board-certified sleep physician.
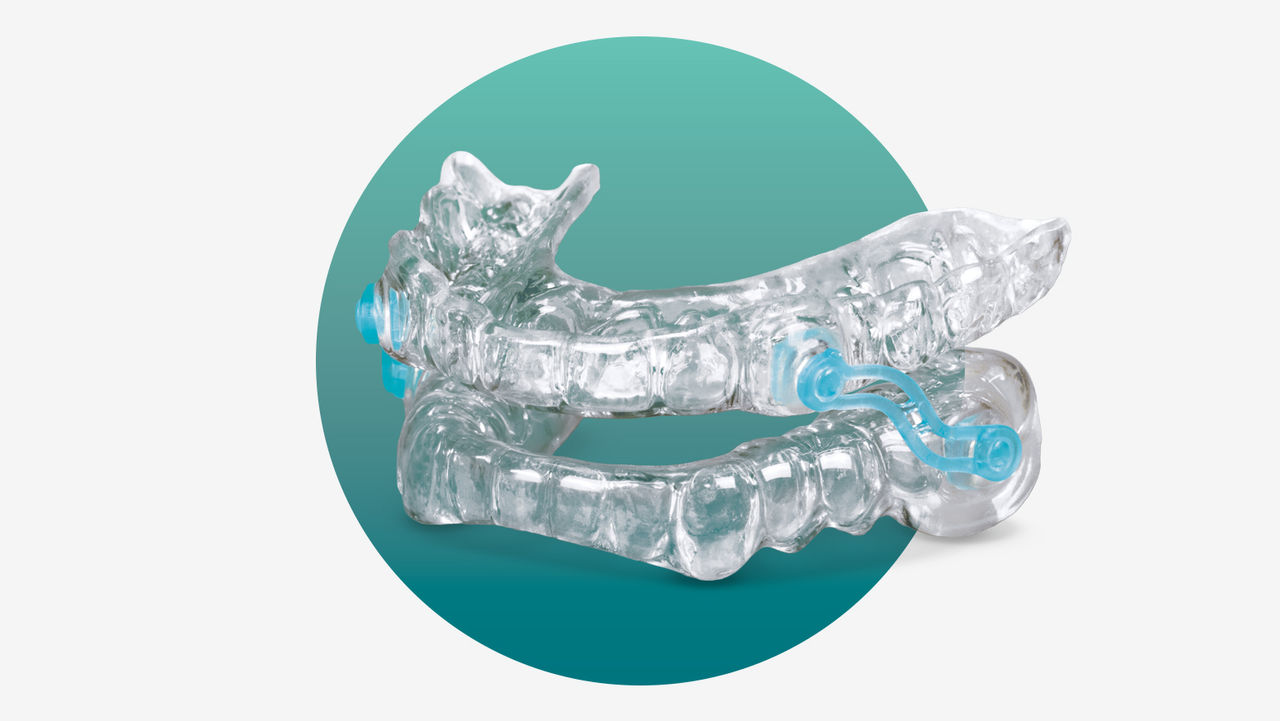
The Silent Nite Sleep Appliance treats snoring and sleep apnea quickly and effectively.
The Silent Nite Sleep Appliance is a mandibular advancement device that has been used to treat patients for snoring since 1997. In 2019, the FDA cleared the Silent Nite appliance for the treatment of mild to moderate obstructive sleep apnea. Fabrication of the Silent Nite appliance requires an upper and lower impression and an edge-to-edge or comfortable protrusive bite registration. This sleep appliance is adjustable up to 6 mm forward from the starting position by changing the length of the connector arms on the sides of the appliance. The adjustment of the appliance puts lateral tension on the muscles and ligaments of the upper airway to help provide tension on the tissues and splint the airway open to prevent the collapse that is OSA.
The EMA oral appliance is FDA-cleared for the treatment of snoring and sleep apnea. The EMA has 36 elastic straps of different lengths and elastic tension options. It is important to note that when using the EMA device, the elastic straps must be changed on a monthly basis to ensure that the jaw is held in the prescribed position all night, every night.
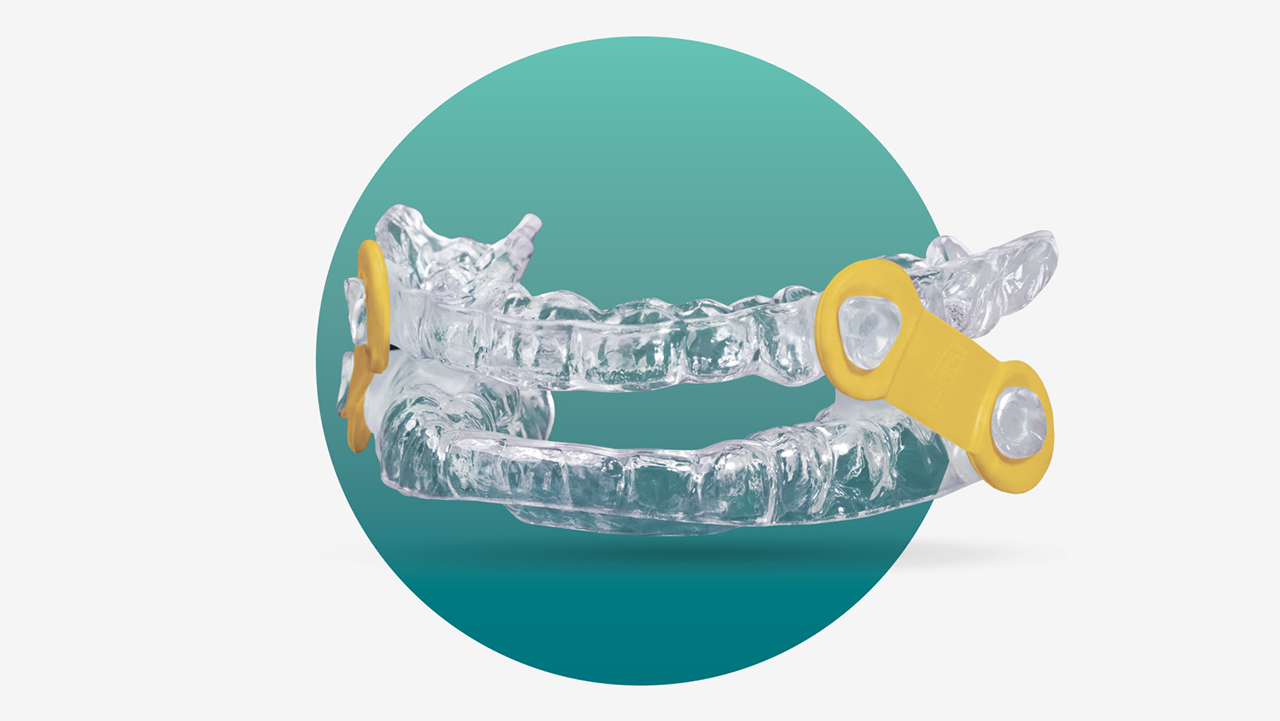
The EMA appliance promotes a deeper, more restful sleep by preventing snoring and relieving the symptoms of obstructive sleep apnea.
Patients treated with the EMA appliance should be informed that they may have sleep apnea, and they should be referred to their primary care physician or a board-certified sleep physician for investigation, diagnosis and management of their sleep condition, which may or may not be sleep apnea. This can take some time, so it is prudent to treat the patient provisionally while the diagnostic process is underway. PMAD therapy should be documented in the patient’s medical record using the informed consent document.
To discover the simple, three-step process to screen and treat patients for snoring and sleep apnea, visit glidewelldental.com/pmad.
REFERENCES
EMA is a registered trademark of Frantz Design Inc.
Send blog-related questions and suggestions to hello@glidewell.com.