Dental CE
- Free on-demand CE course by Mark E. Abramson, DDS: “Introduction to Dental Sleep Medicine”
Related Dental Article
- Randy Clare, Glidewell: “How to Select the Best Anti-Snoring Solution for Your Patient”
800-854-7256 USA
Many issues contribute to a recall remediation. Patient needs are the driver.
Patients who suffer from sleep apnea often rely on nightly therapy to maintain a healthy, active lifestyle, and many patients depend on a continuous positive airway pressure (CPAP) machine. In response to FDA inquiry, Philips Respironics has recently recalled about 4 million respiratory devices, leaving patients with difficult choices and few options.
Dentists have a solution that will serve the needs of patients who suffer from mild to moderate sleep apnea. In this article, we will explain how the recall may affect your patients and how some of these patients may be served in the dental office.
The most effective treatments for sleep apnea in terms of number of treatments provided and access to those treatments are CPAP, bilevel positive airway pressure (BiPAP), and automatic continuous positive airway pressure (APAP). This class of respiratory therapy is also simply called positive airway pressure (PAP).
Dental treatments such as oral appliance therapy (OAT) are well supported in the literature for mild to moderate obstructive sleep apnea (OSA), but far less commonly prescribed than PAP. Dentists report that OAT is often omitted from patient consultations by physicians.
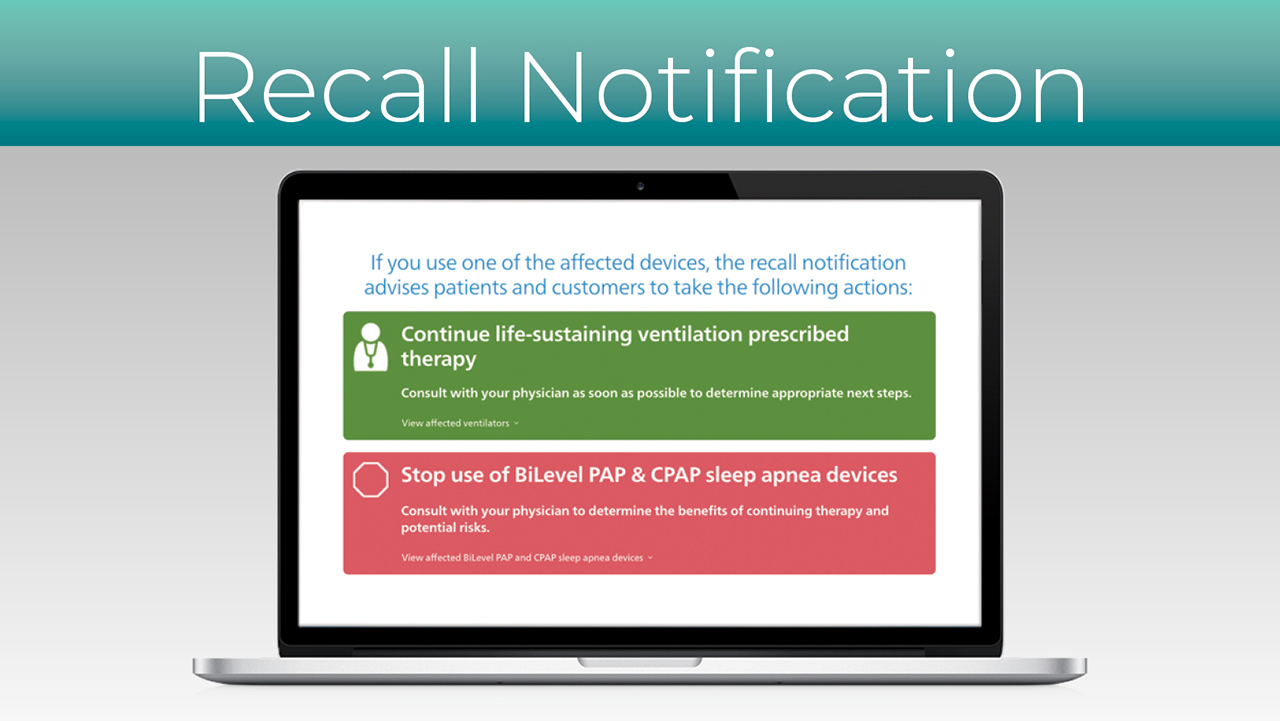
In June 2021, Philips Respironics announced a recall for some 4 million PAP devices, creating a real challenge for patients who use a PAP device to breathe while they sleep. Philips has issued an advisory for patients to “discontinue use of your device and work with your physician or durable medical equipment (DME) provider to determine the most appropriate options for continued treatment.”
It is important to understand what devices are being recalled and why, as well as the real-world implications of trying to replace or repair 4 million PAP devices. This recall decision was not made lightly. Possible health risks are associated with continued use of these devices.
The FDA, in its Philips Respironics CPAP recall FAQ, states:
The polyester-based polyurethane (PE-PUR) sound abatement foam, which is used to reduce sound and vibration in these affected devices, may break down and potentially enter the device’s air pathway. If this occurs, black debris from the foam or certain chemicals released into the device’s air pathway may be inhaled or swallowed by the person using the device.
Philips Respironics reported the following possible side effects to the FDA, which spurred the recall initiative:
Philips has received reports of possible patient impact due to foam degradation. The potential risks of particulate exposure include headache, irritation, inflammation, respiratory issues, and possible toxic and carcinogenic effects. The potential risks of chemical exposure due to off-gassing include headache, irritation, hypersensitivity, nausea/vomiting, and possible toxic and carcinogenic effects. Philips has received no reports regarding patient impact related to chemical emissions.
The brands associated with the Philips recall are some of the most popular CPAP and BiPAP devices ever sold. The company estimates that the recall has affected millions of patients.
The recalled BiPAP and CPAP machines include the following:
Continuous ventilator, non-life-supporting devices:
Noncontinuous ventilators:
Philips Respironics recommends discontinuing use of these devices. Patients who have used these devices should contact a doctor and discuss alternatives.
One of the challenges that has presented itself for the $3.9 billion sleep apnea device industry in the face of this recall is that the supply of CPAP and bilevel devices is completely maxed out. Patients who have been successfully treated with CPAP are reporting that they cannot find replacement equipment due to shortages associated with global supply issues and unreasonable spikes in demand driven in large part by this recall.
This is a tragic outcome for patients who wear their CPAP every night to manage or avoid comorbid conditions such as diabetes, stroke and heart disease. The simple act of not sleeping well can affect quality of life, leading to excessive daytime sleepiness, poor job performance and traffic accidents. For example, according to Frost & Sullivan, in 2015 untreated OSA in the U.S. resulted in a loss of productivity estimated at $86.9 billion, and the total economic impact of all motor vehicle accidents in the U.S. where undiagnosed OSA was a contributing factor was estimated at $26.2 billion.
In the September 2021 issue of HME News, Philips CEO Frans van Houten reported that CFR 806 applications have been filed with the FDA. The approval process typically takes eight weeks. Van Houten explained, “We have already produced repair kits and we’ve also even placed stocks in bonded warehouses in a few countries in the world so that we can move quickly out of the gate.”
Source: U.S. Food and Drug Administration. Recalls, corrections and removals (devices). 2020 Sep 29.
Interruptions in sleep apnea treatments are very damaging to the sleep-related health of patients who use them. The consequences of untreated OSA are clear and well understood by patients and clinicians alike.
The uncalculated human cost of this recall is that some patients who have recently received a Philips CPAP device may fail to meet treatment adherence requirements of most medical carriers, resulting in reimbursement denials, termination of care or, worse, having to start the qualification process all over again.
Medicare CPAP regulations allow a 90-day trial period with a CPAP machine to establish compliance, a statistic that is used in order for Medicare to pay for the CPAP device. Compliance is achieved by using the CPAP machine at least four hours per night for 70% of the nights of the trial. Most CPAP machines have a data card or cloud-based data recorder to measure usage and establish compliance.
Compliance is then reported to Medicare following validation of the patient’s compliance data and a face-to-face meeting with a clinician to confirm that therapy is effective. If after three months the patient did not achieve compliance, as may be the case with the Philips recall, Medicare will not cover the cost of the device or the supplies. In order to restart the process, another face-to-face evaluation with a physician is required and the patient starts the process again as a “new patient.”
The impact of this policy on patient care may result in a temporary halt to this practice, during Philips recall remediation; however, at the time of this writing there has been no announcement by the Centers for Medicare and Medicaid Services (CMS).
Each private insurer sets its own standards for acceptable compliance criteria. While it is very common for private insurers to follow Medicare guidelines regarding adherence, this is not always the case. A call to the patient’s provider is essential to confirm these details.
The scale of the possible number of patients affected by the recall is extremely large when we consider that, in addition to the existing installation base, new patients are being diagnosed every week.
Fortunately, Philips has ramped up production of repair kits for the recall — along with CPAP machines like DreamStation 1 and DreamStation 2 devices — to an estimated 80,000 units per week in Q4 of 2021. An estimated 3.5 million to 4 million CPAP machines are to be manufactured by Philips in 2021. In this same period, ResMed, a global manufacturer of PAP devices, is experiencing tremendous growth in its global business. ResMed CEO Mick Farrell reports that its CPAP device sales in the U.S., Canada and Latin America have increased over 30%.
In HME News, Farrell said, “It’s (Philips’) job to focus on the replacement or recall, and we’re focused on the new patients — both together will create unprecedented demand.”
There have been seismic implications of this recall on sleep apnea treatment providers, including dentists. Patients have questions and concerns, and many of them are seeking alternatives to CPAP, as they take greater responsibility for their own care — if only until they can achieve remediation of their compromised CPAP equipment. For many patients, remaining untreated is simply not an option.
OAT is an OSA treatment provided by a dental office for patients who have been diagnosed with mild to moderate sleep apnea, or for those who cannot tolerate or are unable to use CPAP for any reason.
The diagnosis of OSA must be made by a physician using conventional sleep diagnostic protocols, including in-lab polysomnography and in-home unattended level 3 sleep diagnostics. The diagnostic protocol varies by state and medical insurance carrier.
OAT is provided in a dental office and all issues related to the teeth, jaws, soft tissue, and tongue are managed by the dentist. Medical management of OSA remains the responsibility of the patient’s physician or sleep specialist. This manner of treating OSA has been available for many years and is well supported by medical and dental academies. The American Academy of Sleep Medicine and the American Academy of Dental Sleep Medicine have established a guideline for the treatment of sleep apnea and snoring with OAT.
While dental devices (i.e., mandibular advancement devices [MAD]) are very different from PAP in the way they defend the airway from partial or complete collapse, patients who respond well to oral appliances tend to be much more compliant with care. For example, authors of one study noted: “Sleepiness, nocturia, and sleep-related parameters were similarly improved and more patients preferred MAD. As MAD and CPAP showed similar effects on cardiovascular outcome and symptomatic relief even with a comparable length of usage, we might expect MAD as an alternative treatment option for CPAP in this range of OSA group.”
PAP devices are finely tuned medical-grade blowers that project air into the airway at a specific and therapeutic pressure that is established during a sleep study. This column of air is delivered through a hose from the bedside table to a mask the patient wears. The air trajectory forms a pneumatic splint holding the airway open.
The air pressure can be increased to accommodate any degree of airway collapse, which is why CPAP therapy is so important for patients with more severe disease or who do not respond to OAT.
An oral appliance has a much narrower method of action than CPAP. The device consists of a simple upper and lower nightguard joined in a way that allows the jaw to be held slightly forward while in use. Modern mandibular advancement devices can be adjusted in 1 mm increments to find the optimal jaw position for a patent airway, patient comfort and impact on OSA severity.
MADs work based on a fundamental physiological principle. The tissues, muscles, and ligaments of the oropharynx and the tongue are attached to or affected by the position of the jaw. In very simple terms, moving the jaw forward with a MAD and holding it there during sleep puts lateral tension on these structures, which splints the airway open and holds the tongue forward so that the airway is less prone to partial or complete collapse.
Source: Adapted from Viviano J. OSA appliance anatomy and closing the deal. Slides presented at: Greater New York Dental Meeting. 2019 Dec 4. New York City, New York.
MADs like the Silent Nite® Sleep Appliance, EMA® appliance, OASYS Hinge Appliance™, dreamTAP™ and TAP® 3 TL, which are available from Glidewell, all achieve this effect, but in different ways:
All of these patient-specific oral appliances can be made in any dental office with supplies that are customarily used by a dentist. These devices are typically adjusted after delivery, so it is very uncommon to require a remake for fit or clinical reasons.
For the general dentist with an interest in sleep apnea therapy, there are two takeaways from the tragic impact of the Philips Respironics CPAP recall.
First, the recall is so large that the impact on distribution due to supply chain issues, contractual obligations to buying groups, and clinical organizations’ remediation will take time and may not resolve as quickly as initially anticipated.
Many U.S. communities will be touched in some way by this recall. Patients who have been diagnosed with sleep apnea may feel a significant amount of stress associated with discontinuing their care. Compliant patients are fully aware of the difference between how they feel when they are treated and how they feel when they skip a night, such as when camping or when a storm disrupts power.
This recall was issued in June 2021. With expected FDA filings completed in September, approvals cannot come sooner than November. If the rollout is seamless, we may see remediation complete by Christmas 2022. This is a long time to go untreated, and for many patients and clinicians it is unacceptable. They will be forced to decide if the risk of using a device that was recalled due to a measurable health risk is less than the health implications of returning to their pretreatment lifestyle — which could potentially include nodding off in meetings, falling asleep in front of the TV, or struggling to stay awake at the wheel of the school bus they drive at work.
Second, there are about 140,000 dentists in the U.S. who can participate in the care of OSA patients, and over 50% of adults see their dentist twice per year, according to the American Dental Association. At the very least, dentists can help advise these patients about the qualification criteria for MAD and help those who qualify get treated. Most patients do not realize it, but dentists can fill sleep apnea prescriptions by physicians with MAD devices, and Medicare, Medicaid and private medical insurers will reimburse for the care.
MAD therapy is not new to dentistry, and the techniques, materials and equipment required are all readily available to a practicing dentist. Addressing the need for sleep apnea treatment in light of the Philips Respironics recall is a big responsibility. It affects a significant number of patients and will require a multidisciplinary approach. It will require coordination by physicians, DME providers and dentists. Our patients require this of us.
Dental CE
Related Dental Article
Send blog-related questions and suggestions to hello@glidewell.com.


