AlloDerm®: An Effective Alternative to Palatal Donor Tissue for Treatment of Soft Tissue Alveolar Ridge Defects
When I was in dental school, I did a graft on my cousin and fellow dental student, David, with the help of one of our instructors. David was getting ortho done at the school and had minimal attached gingival on the facial of an upper bicuspid. Since the archwire was going to slightly expand his upper arch, the graft was necessary prior to starting the orthodontics. Cutting into your cousin’s palate with a Bard-Parker in the first year of clinical dentistry was a little scary for me. Anesthetizing the entire left hand side of his palate was a little out of my comfort zone as well. However, both of those paled in comparison to watching him try to eat and drink in the days afterwards since we were roommates. He had a lot of pain in that next week, especially when the perio pack fell off about five minutes after the surgery. Dr. Edward Allen shows an elegant method of avoiding using donor tissue from the palate when performing a graft. I can think of many patients who have refused this type of esthetic periodontal surgery because of the palatal surgery, and the option Dr. Allen demonstrates allows patients to have the graft done without the palatal procedure.
Soft tissue grafting using palatal donor tissue, primarily to increase the zone of attached gingiva, was introduced more than 40 years ago.1 Subsequently, there have been numerous reports describing use of palatal connective tissue grafts for treatment of gingival recession defects and soft tissue alveolar ridge defects.2-14 Surgical applications of soft tissue grafting have been improved by the introduction of microsurgery with more precise incisions, closer wound apposition, reduced hemorrhage and reduced trauma at the surgical site.15 Microsurgery uses smaller instruments and smaller suture, providing less invasive surgery and less complicated postoperative sequellae.
Additionally, in patients who seek treatment, anatomical or medical concerns may be present that rule out use of the palate as a donor source. For these reasons, an effective substitute for palatal donor tissue is necessary.
In spite of significant refinements in surgical technique, many patients remain fearful of palatal surgery and resist recommendations for treatment involving soft tissue grafting.
AlloDerm® Regenerative Tissue Matrix
AlloDerm® (LifeCell Corporation; Branchburg, N.J.), widely used in both medical and dental surgery over the past 12 years, is an acellular dermal matrix derived from donated human skin tissue supplied by tissue banks in the United States, utilizing the standards of the American Association of Tissue Banks and Food and Drug Administration (FDA) guidelines. Human skin consists of both epidermis and dermis. In nature, the dermis contains a framework of cells and structural components that allow it to regenerate and replace itself continually throughout life.
After determining that donated skin tissue is eligible for transplantation, LifeCell processes the tissue. When AlloDerm is prepared, the human donor tissue undergoes a multi-step proprietary process that removes both the epidermis and the cells in the dermis that can lead to a recipient response, tissue rejection and graft failure, without altering the structural and biochemical components of the matrix. The resulting extracellular matrix is freeze-dried by a proprietary method to preserve the tissue without the formation of damaging ice crystals. This entire process is performed under stringent documented quality-controlled systems that have been demonstrated to reduce HIV-l and the surrogate for hepatitis C virus to nondetectable levels (>99.9%). Histology testing is completed on each lot of final product to verify cell removal. Cell removal ensures against viral replication and renders free particles more susceptible to detergent inactivation. In addition to viral safety, both incoming tissue and final products are screened for bacterial and fungal growth and deemed negative. The remaining fibrous framework of biologically active components and vascular channels stimulates the recipient to initiate the intrinsic tissue regeneration process, integrating and replacing the graft tissue with newly formed dense, collagenous connective tissue.16,17,18 Thus, AlloDerm provides a viable biologic substitute for palatal donor tissue.
Use of AlloDerm in Periodontics
AlloDerm is used as a substitute for palatal connective tissue in the treatment of gingival recession and soft tissue alveolar ridge defects. Randomized controlled clinical trials have demonstrated that root coverage with AlloDerm is equivalent to that obtained with palatal donor tissue in the treatment of gingival recession.18-22 An increase in marginal tissue thickness equivalent to palatal tissue grafts has been demonstrated at six and 12 months postoperatively, by both clinical and histometric analysis.18,21 Equivalent attachment to the root surface has been found by histologic evaluation of human bock sections at six months postoperatively.18 Case reports have demonstrated effective restoration of buccal alveolar ridge defects with AlloDerm grafts.23,24 A recent case series described the successful repair of oronasal fistulae with AlloDerm grafts.25 This report describes techniques for using AlloDerm in the treatment of one- and two-tooth buccal alveolar ridge defects.
Ridge Augmentation Surgical Technique
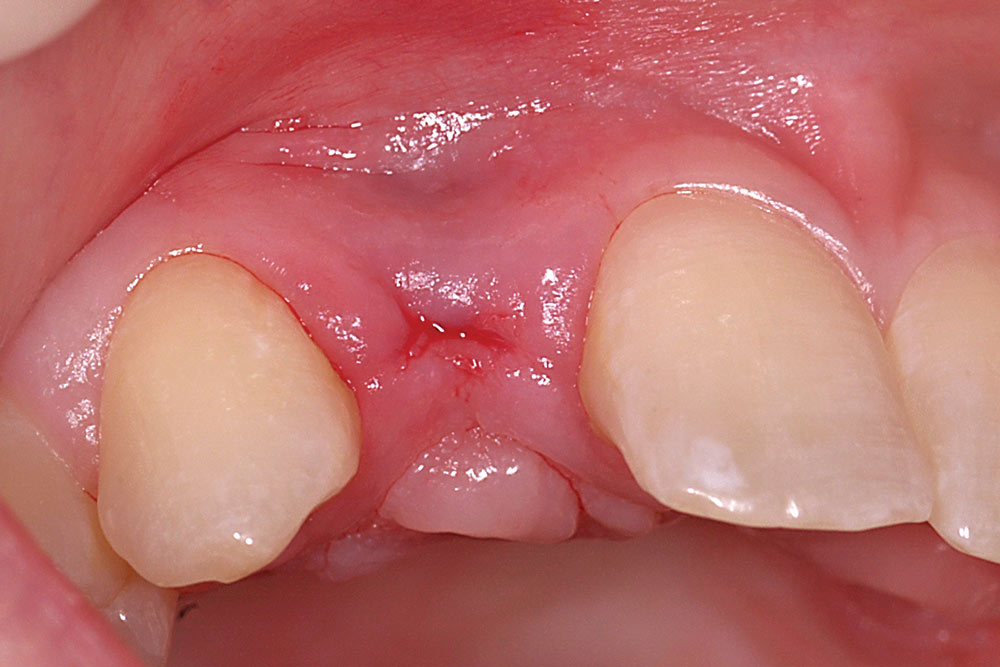
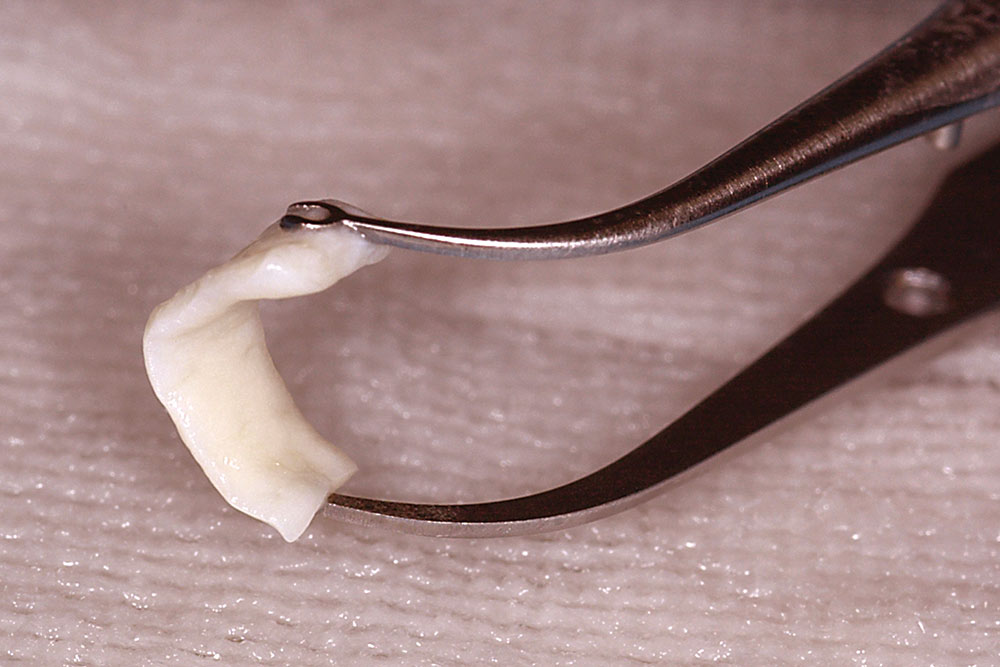
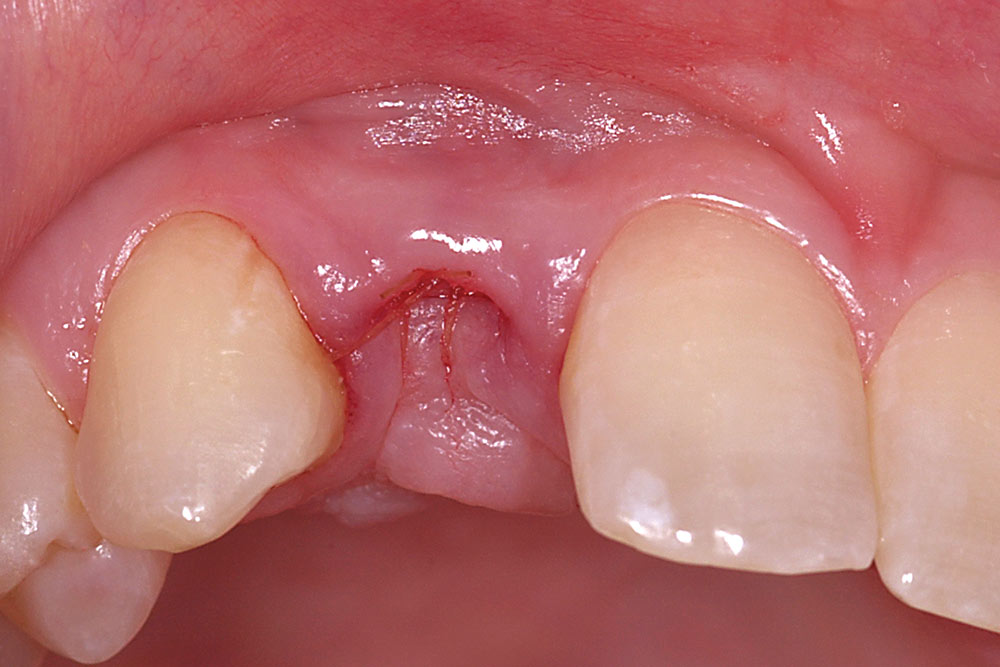
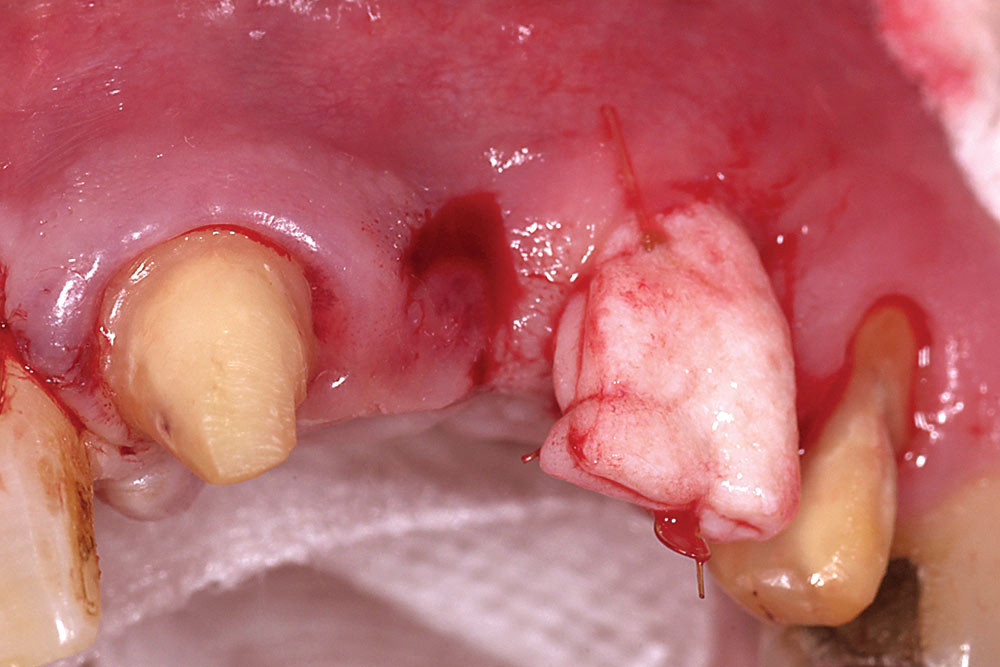
For a single missing tooth site, place a semilunar incision with a #15 blade beginning at the deficient ridge crest, curving facially; do not cross over the papillae into sulci of the adjacent teeth (Fig. 1). This incision should extend to the bone surface. Create a facial pouch in the defect area by sharp supraperiosteal dissection, extending approximately 6–10 mm apical to the ridge crest. Place incisions in the proximal sulcus of each adjacent tooth to mobilize the papillae. Measure the width and depth of the pouch, approximately 6–10 mm horizontally and 8–10 mm vertically. Following two successive washes in normal saline, the AlloDerm is trimmed to create a graft of proper width and a length two times the depth of the pouch.
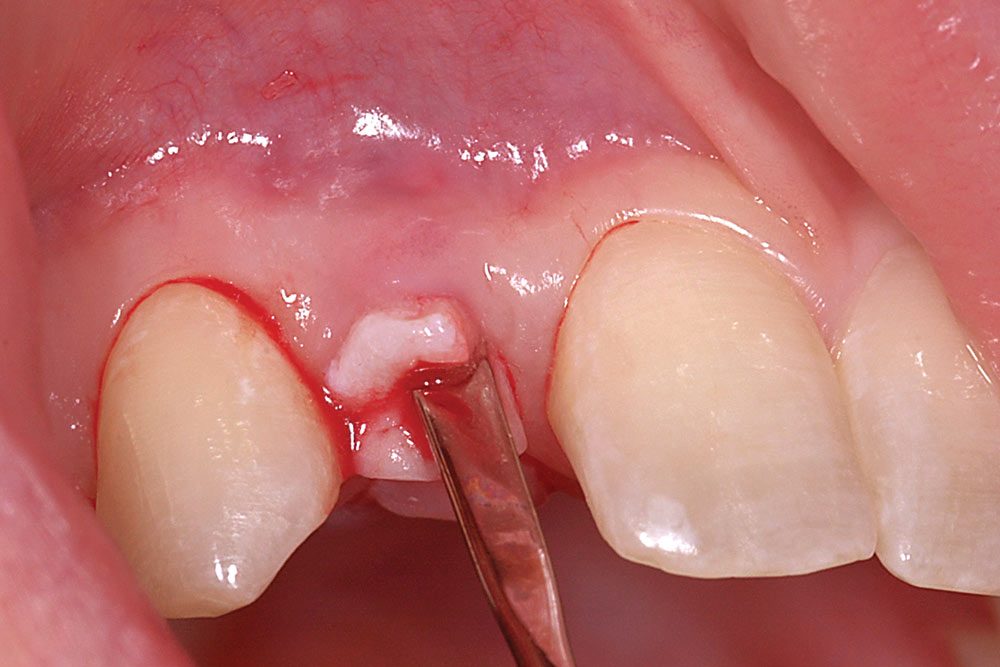
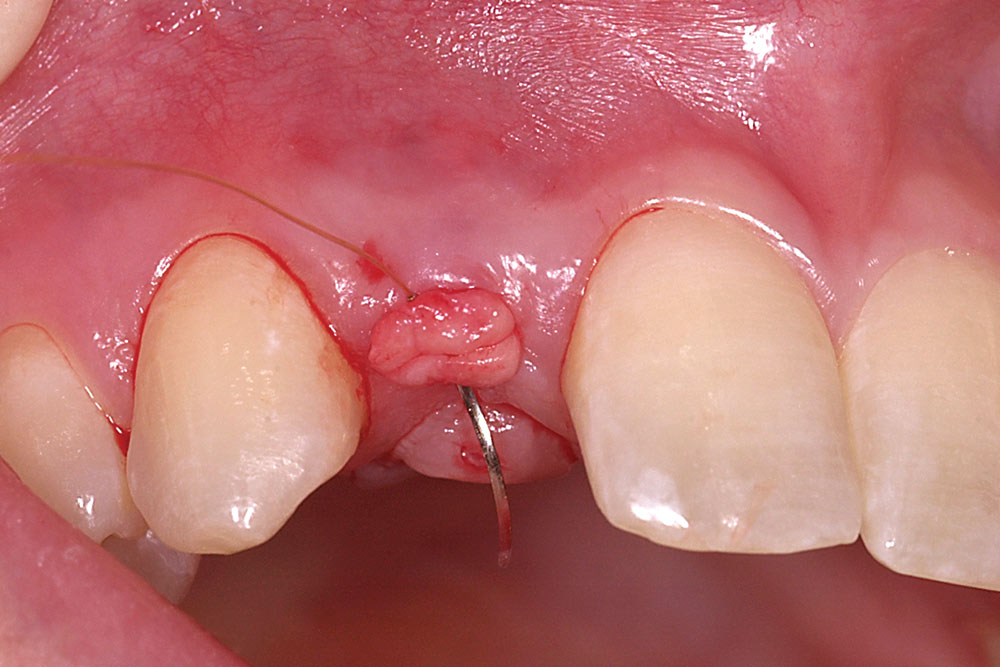
Fold the graft in half longitudinally, approximating the basement membrane surface (Fig. 2), and while grasping the approximated ends with Microsurgical Corn Suture Pliers (Hu-Friedy; Chicago, Ill.), introduce the folded end of the AlloDerm graft into the recipient pouch with an Allen Micro-elevator (Hu-Friedy) (Fig. 3). Place an interrupted suture through the two exposed ends of the AlloDerm graft coronally (Fig. 4), recapture the needle, then push the graft ends just under the lip of the pouch. Pass the needle through the palatal wall of the pouch to secure the coronal aspect of the graft (Fig. 5). Replace the provisional fixed partial denture (FPD) with the ovate pontic lightly contacting the graft.
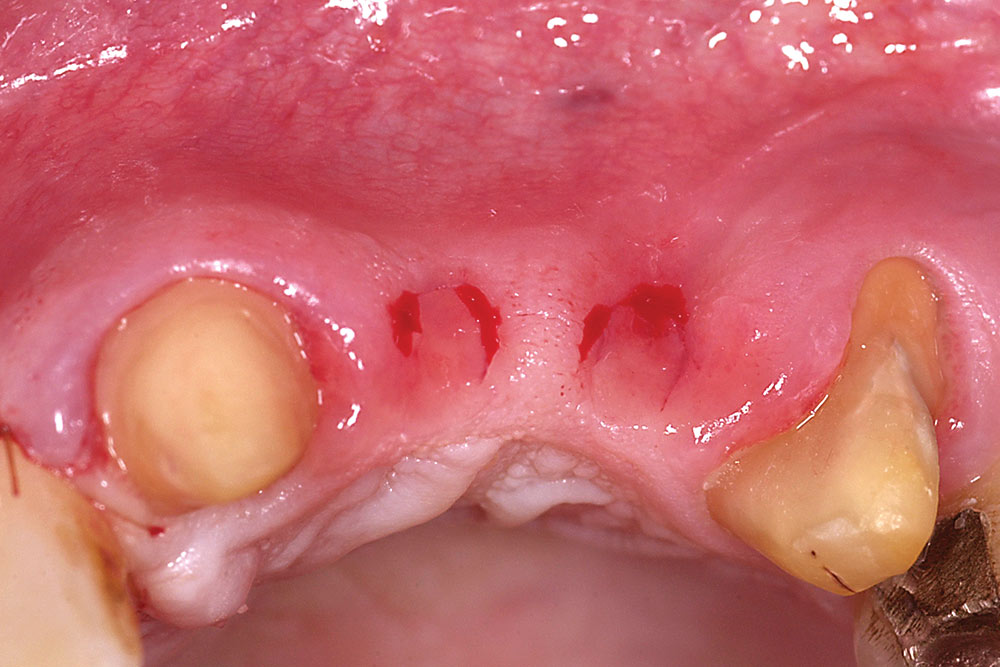
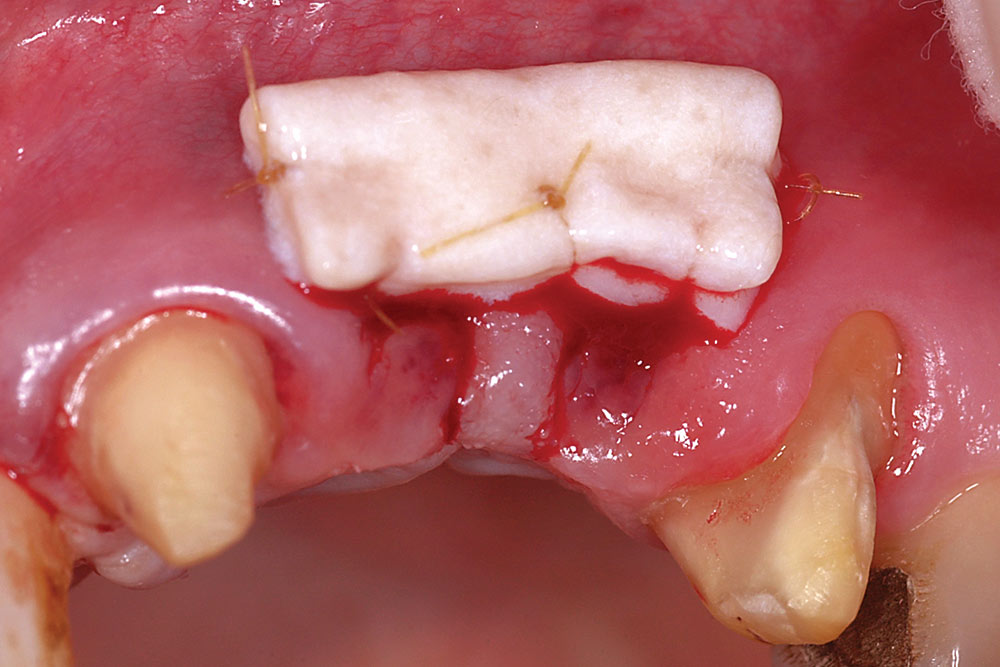
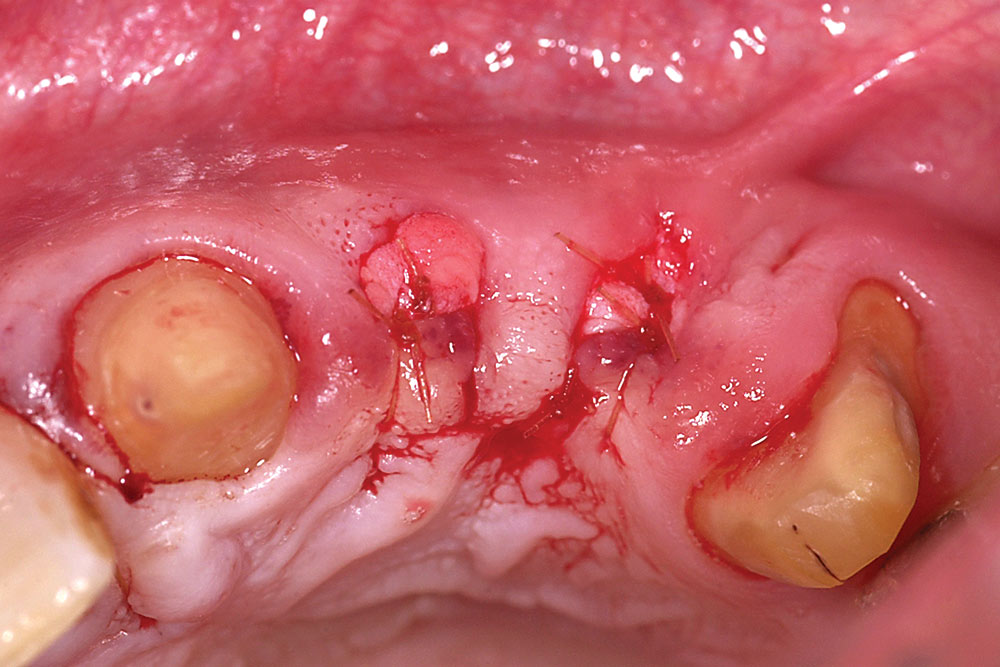
For two adjacent missing teeth (Fig. 6), place a semilunar incision at the ridge crest as described above at the location of each missing tooth, leaving the papillary area between the missing teeth intact (Fig. 7). Create a supraperiosteal pouch by sharp dissection facial to both missing teeth, tunneling under the papillary area. Measure the width and depth of the pouch and trim an AlloDerm graft with a width equal to the span of the two-tooth site and a length twice the pouch depth. Fold the AlloDerm graft in half longitudinally and secure the margins with 6-0 resorbable suture (Fig. 8). Introduce the graft into the pouch through one of the semilunar incisions (Fig. 9), align horizontally within the pouch, and secure the coronal graft border to the palatal pouch wall with 6-0 suture (Fig. 10). Replace the provisional FPD with the ovate pontics lightly contacting the graft (Fig. 11).
Postoperative care includes the following:
- Systemic antibiotics for 10 days
- Chlorhexidine mouthrinse for two to three weeks
- Pain medication, as needed
- Ice applied to face for 24 hours, intermittently, at 10 minute intervals
- Cold liquids for the first three meals
- No mastication or toothbrushing at surgical site for two to three weeks
- Remove surface sutures at two to four weeks
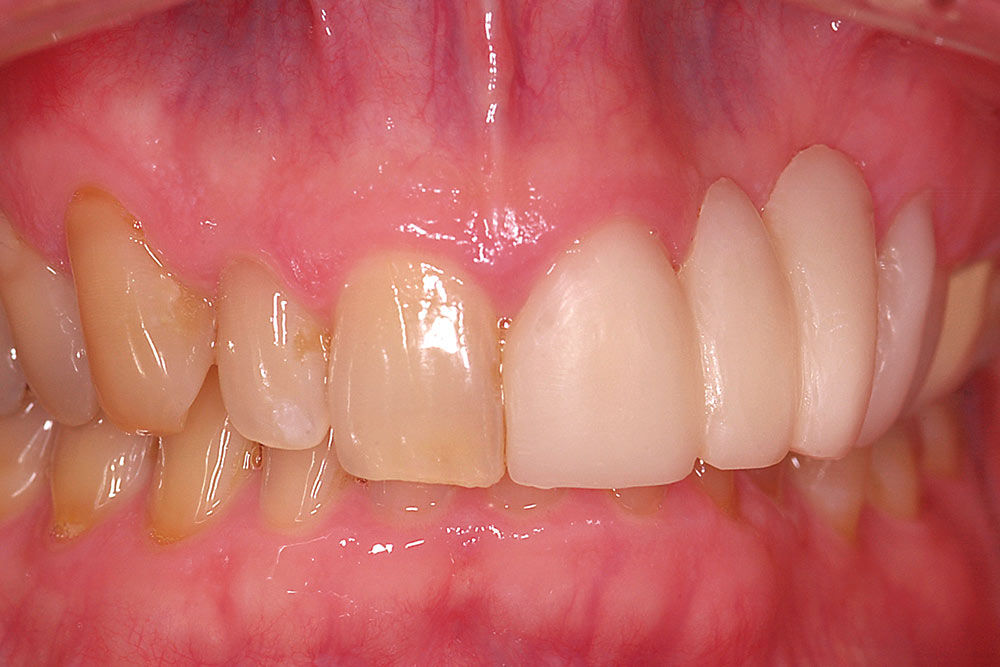
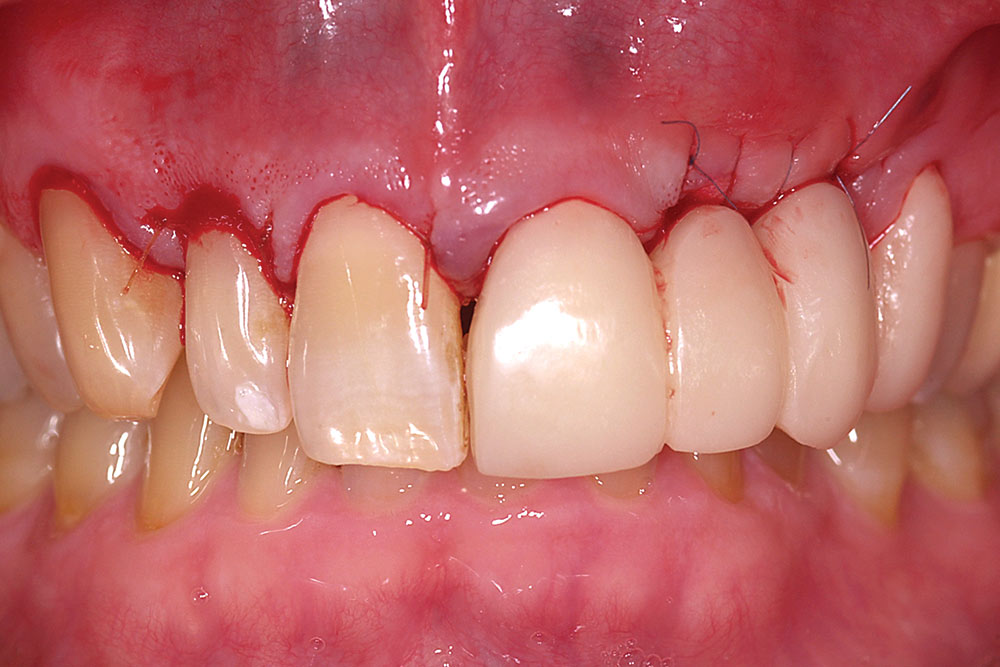
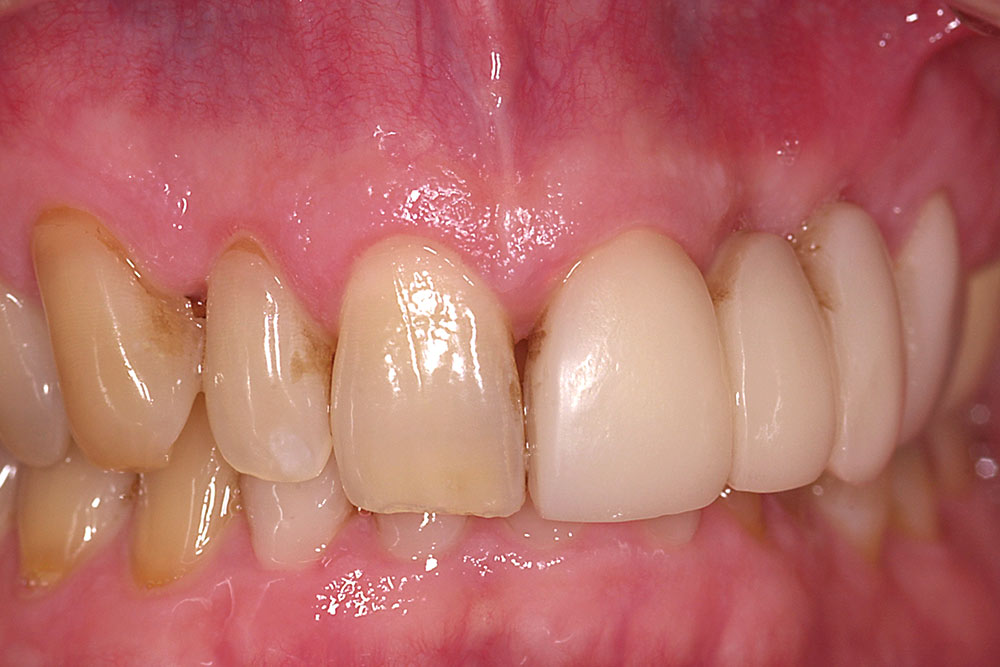
Initial postoperative swelling peaks approximately three days after surgery and gradually subsides over a two-week period. By six to eight weeks postoperatively, the gain of ridge contour will approximate the thickness of the layers of AlloDerm placed (Fig. 12). If additional contour is needed in larger buccal defects, a second augmentation may be performed at eight weeks following the initial procedure. The soft tissue form gained will be maintained long-term provided ovate pontics remain in contact with the restored ridge.
Discussion
Developed more than 10 years ago, AlloDerm is a safe and effective biomaterial for use as a substitute for palatal connective tissue in root coverage grafting and soft tissue ridge augmentation procedures. There have been no reports of disease transmission as a result of AlloDerm use in medical or dental applications. AlloDerm has proven equivalence to palatal connective tissue for root coverage procedures in randomized controlled clinical trials.19,20,21,22 AlloDerm provides distinct advantages over palatal connective tissue in that it does not require a second surgical site to obtain donor tissue, and provides an unlimited amount of tissue to treat multiple sites at one appointment. Patient acceptance of recommended treatment is increased due to elimination of the fear associated with use of the palate for harvesting donor tissue. When compared to the use of palatal donor tissue, the postoperative experience with AlloDerm is less complicated, without the sequellae associated with palatal donor surgery. Further, patients are not as reluctant to return for additional AlloDerm grafting procedures when required.
A successful surgical outcome is dependent on proper pontic and abutment form beginning the day of surgery and continuing through the healing phase. The same is true for the final restorations in order to ensure long-term maintenance of ridge form.
AlloDerm grafting is effective for restoring buccal ridge form at sites of ridge deformities due to developmental defects, trauma or natural ridge resorption. Complete restoration of form is related to the anatomy of the site and the number of contiguous missing teeth. Results are best at sites without loss of proximal bone or soft tissue on the teeth adjacent to the edentulous ridge and at one- and two-tooth sites. A gain in tissue thickness of approximately 3 mm can be expected from a single surgery using a folded AlloDerm graft. At sites with greater than 3 mm of buccal deficiency, a second surgery may be necessary. Minimal increase in vertical dimension can be achieved with the technique described.
Long-term maintenance of the soft tissue form achieved is dependent on the support of the adjacent bony walls proximally and the ovate pontic form crestally. Without the support of the ovate pontic during the healing phase, there will be loss of the coronal portion of the augmented site. Shrinkage of the ridge will occur without the support from the convex form of the pontic maintaining the concave pontic site in the ridge. Such shrinkage is commonly seen where modified ridge-lap pontics are used. A successful surgical outcome is dependent on proper pontic and abutment form beginning the day of surgery and continuing through the healing phase. The same is true for the final restorations in order to ensure long-term maintenance of ridge form.
Conclusion
The surgical techniques outlined represent a refinement in the approach to soft tissue ridge augmentation. The procedures are minimally invasive microsurgical techniques that enhance wound stability while reducing morbidity due to the use of AlloDerm. Reduced discomfort and improved patient satisfaction characterize the postoperative period.
To contact Dr. Edward Allen, call 877-696-1414, email center@epallendds.com or visit dredwardpallen.com.
References
- ^Björn H. Free transplantation of gingival propria. Sveriges Tandlakar forbunds Tidning. 1963;22:684-9.
- ^Miller PD Jr. Root coverage using a free soft tissue autograft following citric acid application. Part 1: Technique. Int J Periodontics Restorative Dent. 1982;2(1):65-70.
- ^Raetzke PB. Covering localized areas of root exposure employing the “envelope” technique. J Periodontol. 1985 Jul;56(7):397-402.
- ^Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985;56:715-20.
- ^Nelson SW. The subpedicle connective tissue graft. A bilaminar reconstructive procedure for the coverage of denuded root surfaces. J Periodontol. 1987 Feb;58(2):95-102.
- ^Allen AL. Use of the supraperiosteal envelope in soft tissue grafting for root coverage. I. Rationale and technique. Int J Periodontics Restorative Dent. 1994 Jun;14(3):216-27.
- ^Azzi R, Etienne D. Recouvrement radiculaire et reconstruction papillaire par greffon conjonctif enfoui sous un lambeau vestibulaire tunnellisé et tracté coronairement. J Parodontol Implant Orale. 1998;17(1):71-7.
- ^Blanes RJ, Allen EP. The bilateral pedicle flap-tunnel technique: a new approach to cover connective tissue grafts. Int J Periodontics Restorative Dent. 1999 Oct;19(5):471-9.
- ^Abrams L. Augmentation of the deformed residual edentulous ridge for fixed prosthesis. Compend Contin Educ Dent. 1980 May-Jun;1(3):205-13.
- ^Garber DA, Rosenberg ES. The edentulous ridge in fixed prosthodontics. Compend Contin Educ Dent. 1981 Jul-Aug;2(4):212-23.
- ^Langer B, Calagna LJ. The subepithelial connective tissue graft. A new approach to the enhancement of anterior cosmetics. Int J Periodontics Restorative Dent. 1982;2(2):22-33.
- ^Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part I. Technique and wound healing. Compend Contin Educ Dent. 1983 Sep-Oct;4(5):437-53.
- ^Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part II. Prosthetic/periodontal relationships. Compend Contin Educ Dent. 1983 Nov-Dec;4(6):549-62.
- ^Allen EP, Gainza CS, Farthing GG, Newbold DA. Improved technique for localized ridge augmentation. A report of 21 cases. J Periodontol. 1985 Apr;56(4):195-9.
- ^Shanelec DA. Periodontal microsurgery. J Esthet Restor Dent. 2003;15(7):402-7.
- ^Silverman RP, Li EN, Holton LH 3rd, Sawan KT, Goldberg NH. Ventral hernia repair using allogenic acellular dermal matrix in a swine model. Hernia. 2004 Dec;8(4):336-42.
- ^Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg. 2004 Feb;52(2):188-94.
- ^Cummings LC, Kaldahl WB, Allen EP. Histologic evaluation of autogenous connective tissue and acellular dermal matrix grafts in humans. J Periodontol. 2005 Feb;76(2):178-86.
- ^Aichelmann-Reidy ME, Yukna RA, Evans GH, et al. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J Periodontol. 2001 Aug;72(8):998-1005.
- ^Novaes AB Jr, Grisi DC, Molina GO, et al. Comparative 6-month clinical study of a subepithelial connective tissue graft and acellular dermal matrix graft for the treatment of gingival recession. J Periodontol. 2001 Nov;72(11):1477-84.
- ^Paolantonio M, Dolci M, Esposito P, et al. Subpedicle acellular dermal matrix graft and autogenous connective tissue graft in the treatment of gingival recessions: a comparative 1-year clinical study. J Periodontol. 2002 Nov;73(11):1299-1307.
- ^Tal H, Moses O, Zohar R, et al. Root coverage of advanced gingival recession: a comparative study between acellular dermal matrix allograft and subepithelial connective tissue grafts. J Periodontol. 2002 Dec;73(12):1405-11.
- ^Fowler EB, Breault LG. Ridge augmentation with a folded acellular dermal matrix allograft: a case report. J Contemp Dent Pract. 2001 Aug 15;2(3):31-40.
- ^Lorson ME, Gapski R. Aesthetic crown lengthening in combination with an acellular dermal matrix for ridge development. Pract Proced Aesthet Dent. 2006 Jul;18(6):371-5.
- ^Kirschner RE, Cabiling DS, Slemp AE, et al. Repair of oronasal fistulae with acellular dermal matrices. Plast Reconstr Surg. 2006 Nov;118(6):1431-40.