A New Lease on Life with the Silent Nite® Sleep Appliance: How Dentists Are Helping Patients Overcome Sleep-Disordered Breathing

Snoring, gasping and choking during sleep, daytime sleepiness and fatigue — these are just a few warning signs of sleep-disordered breathing.1 Although obstructive sleep apnea (OSA) can cause serious health consequences, an estimated 80% of cases are undiagnosed,2 as patients usually don’t realize they have this condition. Fortunately, there is a proven dental solution for sleep-disordered breathing — nighttime mandibular advancement with a device like the Silent Nite® Sleep Appliance. With over 400,000 appliances delivered and more than 20 years since its introduction, the Silent Nite sleep device is a tried-and-true method for treating patients suffering from sleep-disordered breathing.
Advantages of Treating Patients with the Silent Nite Sleep Appliance
✓ More than 400,000 Silent Nite appliances delivered
✓ Introduced in 1997, with more than 20 years of clinical success
✓ Engineered for maximum comfort; adjustable in 1 mm increments
✓ Each case includes an AM Aligner and extra connectors
✓ Treats snoring and mild to moderate obstructive sleep apnea quickly and effectively
Over the years, the appliance has undergone several innovations in response to clinical feedback, and its most recent development occurred in July 2019, when the appliance was cleared by the FDA for an expanded use. Whereas previously, the appliance was used to treat snoring and mild sleep apnea, today, the Silent Nite device is indicated to reduce snoring and mild to moderate obstructive sleep apnea. This expanded indication of moderate sleep apnea makes treatment available to a wider range of patients who might be at risk and offers them a restful night’s sleep so they have more energy during the day.
ORAL APPLIANCE THERAPY
Oral appliance therapy is very effective in treating patients with sleep-disordered breathing, with a compliance rate shown to be as high as 90% over a 2.5-year period.3 With documented clinical success, the Silent Nite appliance works by gently positioning the lower jaw forward during sleep. This mandibular advancement activates the muscles and ligaments of the upper pharynx to establish an unobstructed airway. Each Silent Nite appliance case includes extra connectors, in various sizes, and an AM Aligner to aid patients in exercising and realigning the jaw after nighttime mandibular advancement.

The Silent Nite Sleep Appliance was introduced by Glidewell Laboratories in 1997, and made available to the burgeoning field of dental sleep medicine.
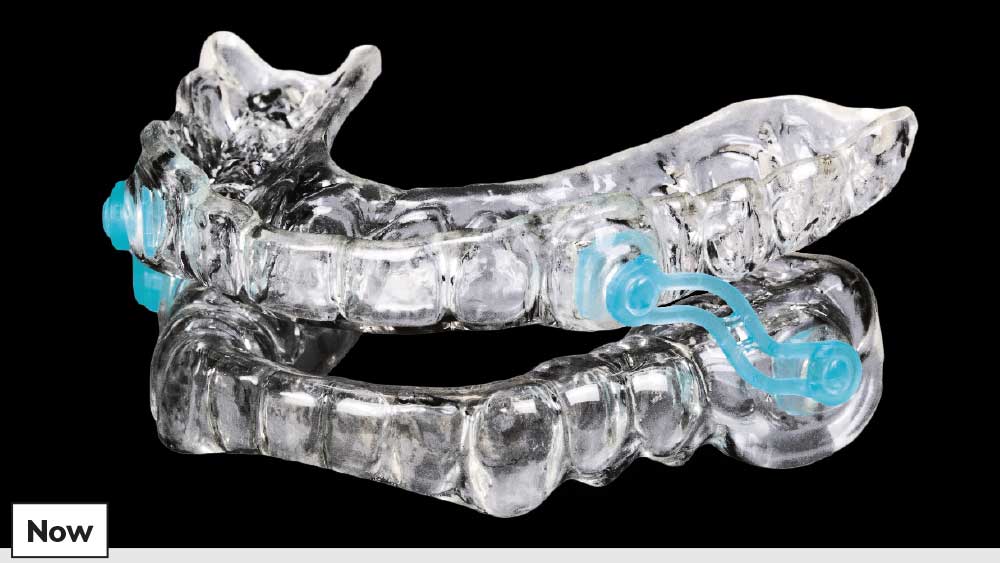
Today, the field of dental sleep medicine continues to grow, and the Silent Nite appliance has become the most popular sleep device from Glidewell Laboratories. More than 400,000 Silent Nite appliances have been delivered to patients, resulting in an extensive amount of clinical feedback used to help maximize treatment success.
The Silent Nite appliance is affordably priced, helping make access to care available to a broad scope of dental patients. Diagnosis of sleep apnea requires a referral to a sleep physician and is critical for these patients. However, with proper informed consent, a provisional diagnosis can be made, and the patient treated on a provisional basis. Dentist-attorney Dr. Ken Berley explains provisional therapy in the article “Legal Perspectives on Oral Appliance Therapy and Dental Sleep Medicine.”
The optimal solution for a sleeping disorder is treatment that the patient will use every night. Because the Silent Nite appliance is flexible, thin and custom-fabricated to be comfortable for all-night wear, it promotes long-term use and patient satisfaction.

Each Silent Nite Sleep Appliance is custom-fabricated in the lab for maximum comfort and all-night wear.
STREAMLINED TREATMENT PROTOCOL
Dental sleep medicine has been identified as one of the fastest growing areas of dentistry.4 For general practitioners, the Silent Nite appliance offers a streamlined approach to treatment and a simplified protocol for provisional therapy.
Step 1: Evaluate your patient for sleep-disordered breathing. Get tips on screening from Dr. Suzanne Haley in the article, “Dental Sleep Medicine: Recognizing and Screening for Obstructive Sleep Apnea in the Dental Practice,” featured in Chairside® magazine (Vol. 12, Issue 2), or learn more by enrolling in a dental sleep medicine CE course at glidewellcecenter.com.

The Silent Nite Sleep Appliance offers many unique features and benefits, including FDA clearance for a new expanded indication of moderate obstructive sleep apnea, and the availability of a free informed consent document that helps dentists introduce immediate treatment.
Step 2: If snoring or sleep apnea is suspected, provide your patient with an informed consent form to initiate provisional treatment, and refer for definitive diagnosis. The legal document “Informed Consent for the Silent Nite Sleep Appliance — Provisional Mandibular Advancement Device” from Dr. Berley provides critical information for both dentists and patients regarding oral appliance therapy. Get it for free at glidewelldental.com/informed-consent. More information about provisional treatment can be found in this article.
Step 3: Treat your patient with the Silent Nite Sleep Appliance. The Silent Nite appliance includes a straightforward treatment protocol, an effort-free bite registration and impression technique, a simple seating appointment, and routine follow-up care. Click here to get started with your Rx.
Key Facts & Figures on Snoring and OSA
- 1 in 4 patients in the dental practice suffers from sleep-disordered breathing.5
- An estimated 80% of OSA cases are undiagnosed.2
- The annual economic burden of undiagnosed sleep apnea among U.S. adults is nearly $150 billion.1
- Untreated sleep apnea increases the risk of costly health complications such as hypertension, heart disease, diabetes and depression.1
- The compliance rate for oral appliance therapy is about 90% over a 2.5-year period.3
- The Silent Nite Sleep Appliance has a 22-year history of clinical success and a streamlined treatment protocol.
CONCLUSION
Studies suggest that even mild degrees of sleep-disordered breathing may be associated with significant morbidity, including excessive daytime sleepiness, long-term cardiovascular complications and substantial societal costs.6 Furthermore, untreated sleep apnea increases the risk of heart attack, high blood pressure, obesity, stroke and diabetes.7 Thus, treatment can positively affect patients’ daytime energy and quality of life. The Silent Nite Sleep Appliance provides an important option for both provisional treatment with informed consent and long-term treatment after definitive diagnosis by a sleep physician. Due to its straightforward treatment protocol, low cost and proven efficacy, the Silent Nite appliance offers many opportunities for dentists to provide reliable care to their patients.
AM Aligner is a product of Airway Management Inc.
References
- ^ Economic burden of undiagnosed sleep apnea in the U.S. is nearly $150B per year [internet]. Darien, Illinois: American Academy of Sleep Medicine; c2019 [cited 2019 July 17]. Available from: https://aasm.org/economic-burden-of-undiagnosed-sleep-apnea-in-u-s-is-nearly-150b-per-year/.
- ^ Sleep apnea information for clinicians [internet]. Washington, D.C.: American Sleep Apnea Association; c2019 [cited 2019 June 17]. Available from: https://www.sleepapnea.org/learn/sleep-apnea-information-clinicians.
- ^ Yoshida K. Effects of a mandibular advancement device for the treatment of sleep apnea syndrome and snoring on respiratory function and sleep quality. Cranio. 2000 Apr;18(2):98-105.
- ^ Haley S. Dental sleep medicine: recognizing and screening for obstructive sleep apnea in the dental practice. Chairside. 2017;12(2):53-60.
- ^ Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015 Apr;3(4):310-8.
- ^ Hosselet J, Ayappa I, Norman RG, Krieger AC, Rapoport DM. Classification of sleep-disordered breathing. Am J Respir Crit Care Med. 2001 Feb;163(2):398-405.
- ^ Sleep apnea: common causes, risk factors, treatments [internet]. Lititz (PA): American Sleep Association; c 2019 [cited 2019 Jul 17]. Available from: https://www.sleepassociation.org/sleep-disorders/sleep-apnea/.


