Enhancing Bone Regeneration with the Use of Platelet Concentrates
In implant dentistry today, many clinicians strive to implement procedures that enhance and accelerate the predictable rates of healing. To increase the regeneration of hard and soft tissues, the use of growth factors is commonly integrated into the surgical protocol. There are more than 50 known growth factors that have been identified in the healing process. Most of the factors enhance the formation and mineralization of bone, induce undifferentiated mesenchymal cells to differentiate into bone cells, and trigger a cascade of intracellular reactions.1 One popular source of growth factors used in implant dentistry is blood concentrates — most specifically, platelets. The platelet, also called a thrombocyte, consists of blood cells that play a crucial role in hemostasis and wound healing. Platelets have a life span of approximately 7–10 days and set the pace of wound repair by releasing their growth factors immediately after the initiation of the clotting process.2 These platelet-derived growth factors have been shown to enhance collagen production, cell mitosis, blood vessel growth, cell recruitment and cell differentiation (Fig. 1).3
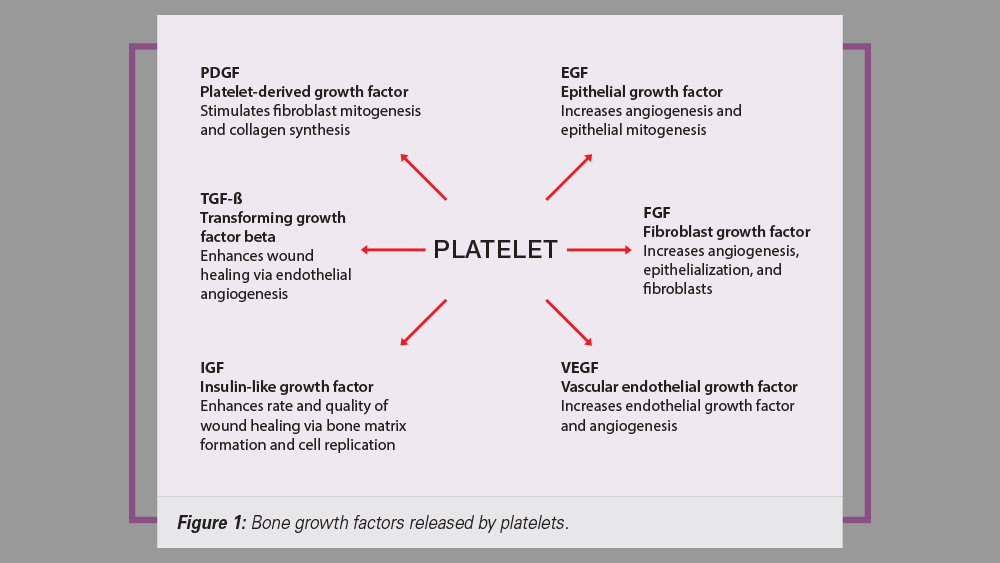
Figure 1: Bone growth factors released by platelets.
PLATELET CONCENTRATE CLASSIFICATION
The two most utilized and studied platelet concentrates in implant dentistry today are platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). The first-generation blood concentrate, platelet-rich plasma, was introduced by Marx in 1998. His studies showed bone maturity to be twice as effective with the use of PRP in grafted sites, and the addition of PRP increased bone density up to 30% in healed sites.4
A second-generation blood substitute, platelet-rich fibrin, was first described by Choukroun in 2001. PRF has been shown to be very effective in the release of important growth factors present in platelets, such as platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-ß), insulin-like growth factor (IGF), fibroblast growth factor (FGF), and epithelial growth factor (EGF).5 Multiple clinical studies with PRF have shown greater soft-tissue healing, enhanced healing of grafted bone, promotion of angiogenesis and faster wound healing.6,7,8 This concentrate has become popular in implant dentistry, as it has a much simpler processing protocol compared with PRP.
PLATELET-RICH CLOT
The PRF clot is a natural-based biomaterial that is obtained from an autogenous blood harvest without the use of anticoagulants or biomedical modifiers. This gel-type fibrin network contains a high concentration of platelets and white blood cells, which release the growth factors at the surgical site.9 The internal organization makeup of platelet-rich fibrin is unique, as it contains three adhesive molecules — fibrin, fibronectin and vitronectin — that result in a highly elastic, matricial mesh architecture. This complex three-dimensional structure allows for a longer release of growth factors, as compared with PRP. As the platelets degranulate, a sustained release of growth factors may range from a time period of one to four weeks.10
DIFFERENCES BETWEEN PRF AND PRP
The PRF clot has become very popular in clinical oral implantology in comparison to the PRP process because it:
- Is naturally polymerized and requires no chemical use (PRP requires a coagulant)
- Requires a conventional, single-spin centrifuge (PRF) vs. two centrifuge spins (PRP)
- Has a slower release of growth factors in comparison to PRP
- Exhibits greater cell migration and proliferation
- Contains a more advantageous fibrin network that stores cytokines and growth factors
- Has better healing properties than PRP
- Requires fewer disposables, reducing cost
PROTOCOL FOR PREPARATION OF PLATELET-RICH FIBRIN
a. Obtaining the Blood Sample
The standard protocol for PRF preparation begins with the venipuncture technique, which involves obtaining approximately 9 ml of blood collected in a sterile, glass-coated plastic tube without anticoagulant (red tube) (Table 1 and Figs. 2–7).
Table 1: Venipuncture Technique
Step 1: Select site for venipuncture (e.g., antecubital fossa, dorsum of the hand or wrist).
Step 2: Place toumiquet approximately 3–4 mm above entry site.
Step 3: Identify the vein location and trajectory. If visualizing the vein is difficult, the following may be used to ease the location of veins: light tapping on the site, warm and moist towel, nitrous oxide, or a vein locator.
Step 4: Clean injection area with alcohol gauze.
Step 5: Venipuncture with a vacutainer, butterfly needle or catheter. Enter tissue and vein to collect the blood sample:
- Pull skin in opposite direction of needle.
- Ensure needle bevel is facing upward.
- Tissue is entered at a 30-degree angle.
- Perforate vein. Then, decrease angle and advance needle slightly.
- Removed touniquet.
- Obtain approximately 9 ml blood collection in a sterile, glass-coated plastic tube without anticoagulant (red tube).
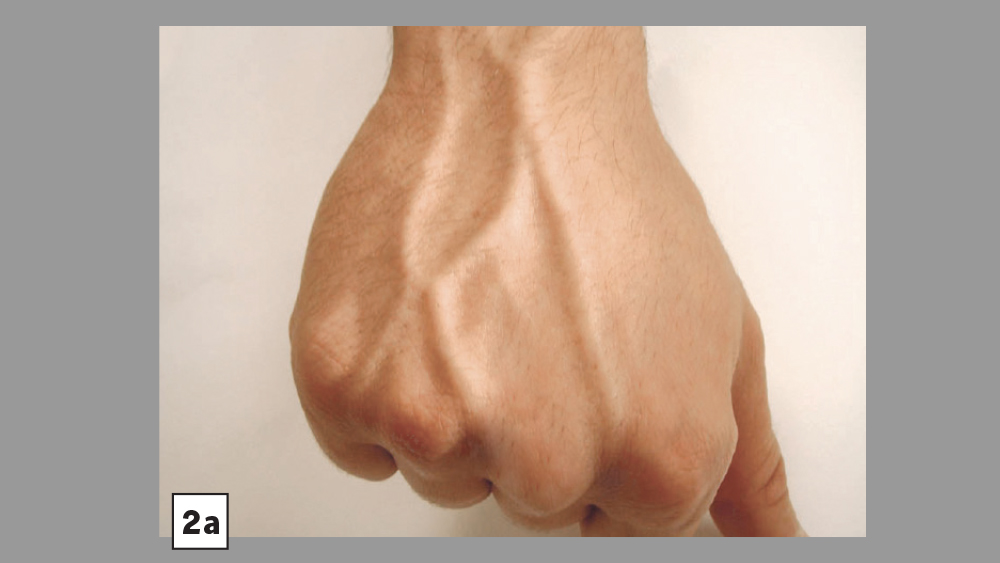
Figures 2a, 2b: Common venipuncture sites include the dorsum of the hand (2a) and antecubital fossa (2b)
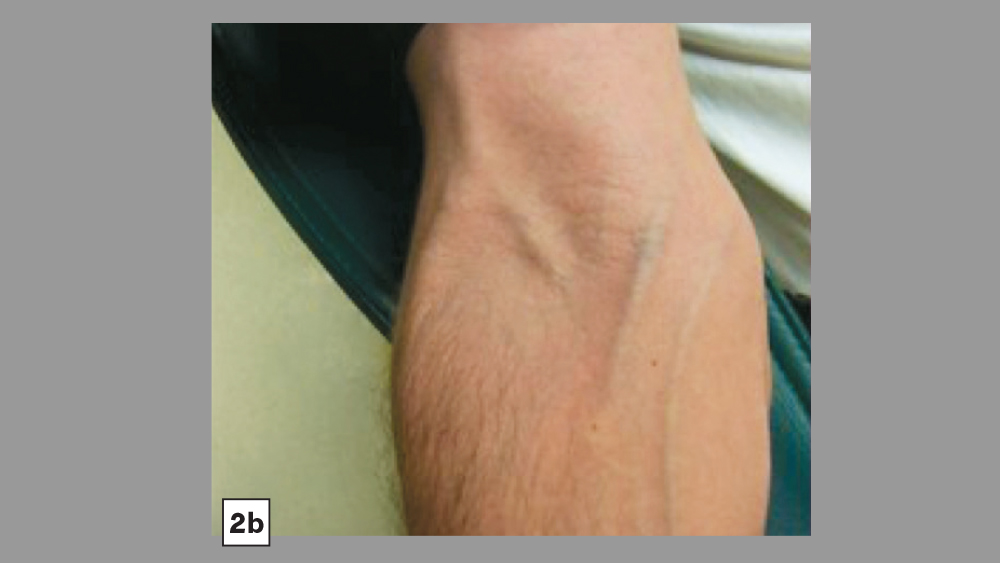
Figures 2a, 2b: Common venipuncture sites include the dorsum of the hand (2a) and antecubital fossa (2b)
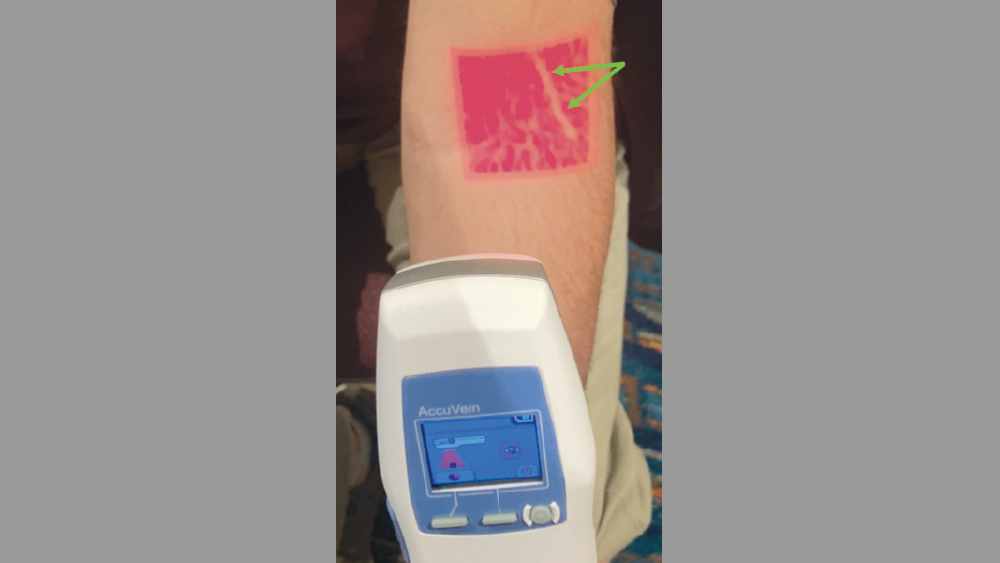
Figure 3: A vein finder allows for ease of locating and determining the trajectory of veins.
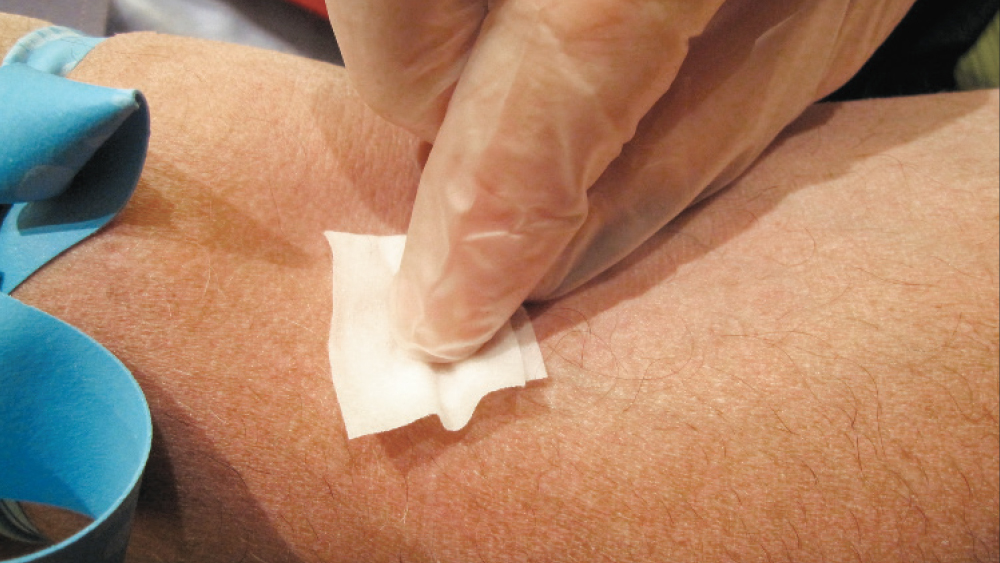
Figure 4: The site is cleaned with alcohol gauze.
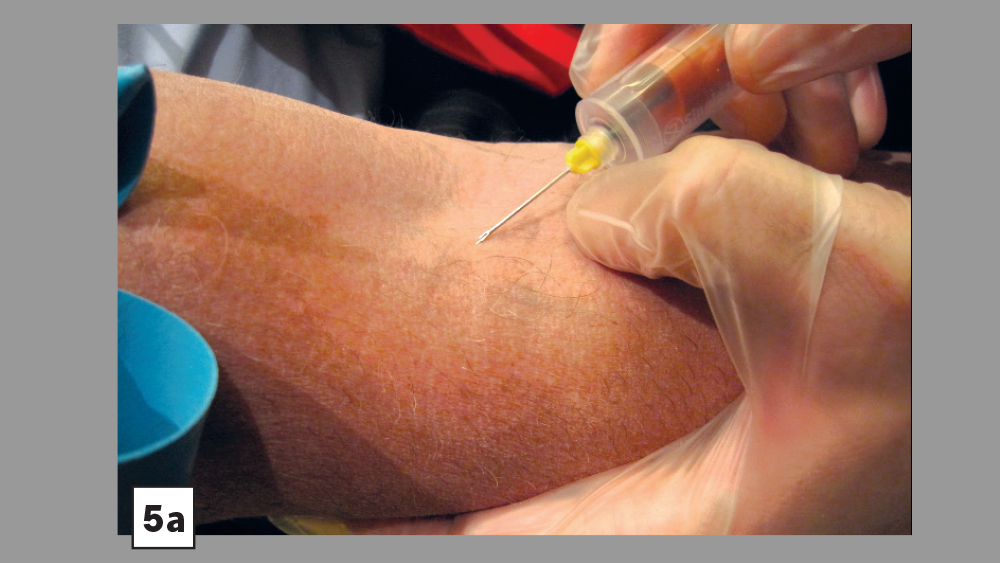
Figures 5a, 5b: Skin is pulled tight and needle is directed at a 30-degree angle (5a). Vein is entered; needle angle is reduced and slightly advanced (Vacutainer® technique) (5b).
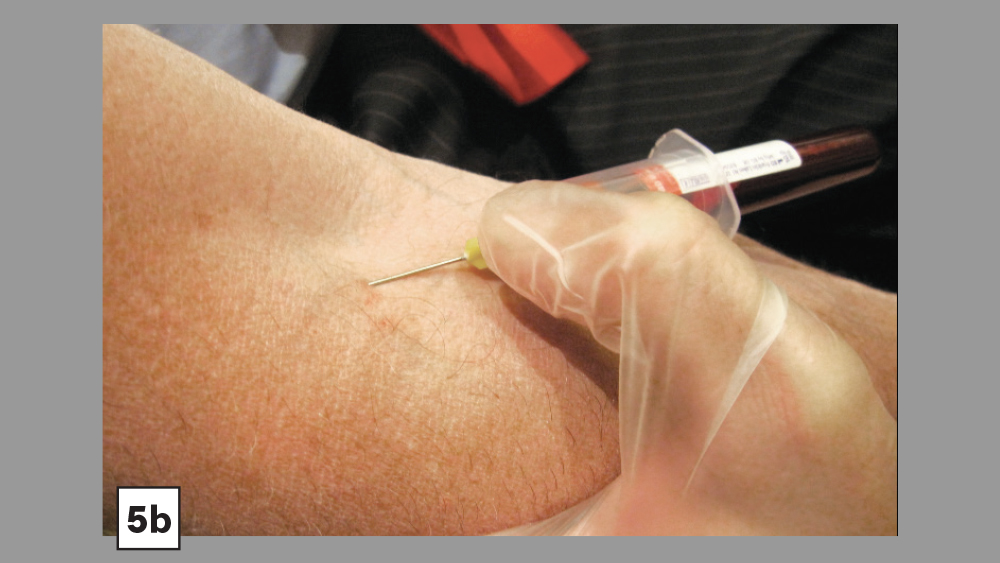
Figures 5a, 5b: Skin is pulled tight and needle is directed at a 30-degree angle (5a). Vein is entered; needle angle is reduced and slightly advanced (Vacutainer® technique) (5b).
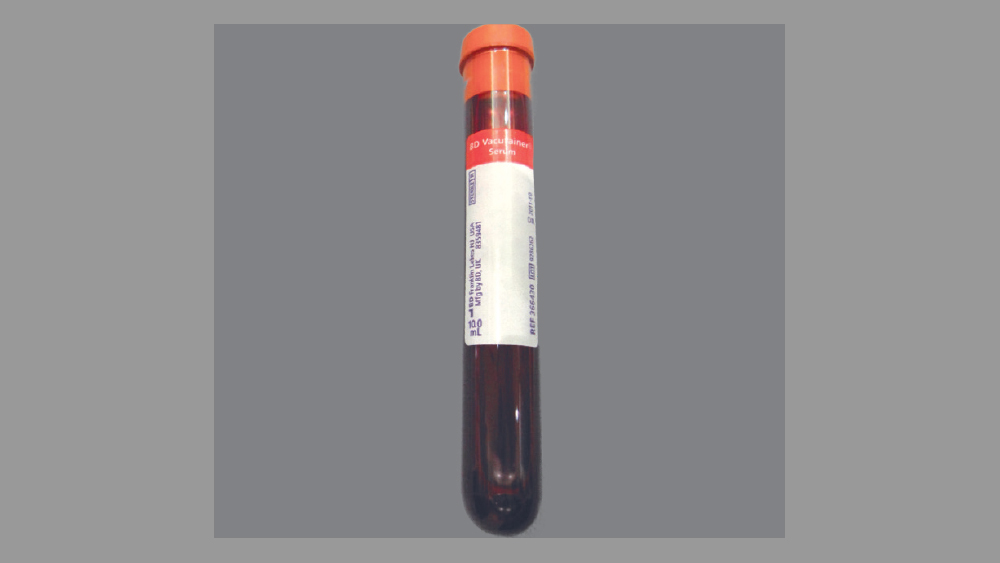
Figure 6: Blood sample is obtained in a sterile tube without anticoagulant.
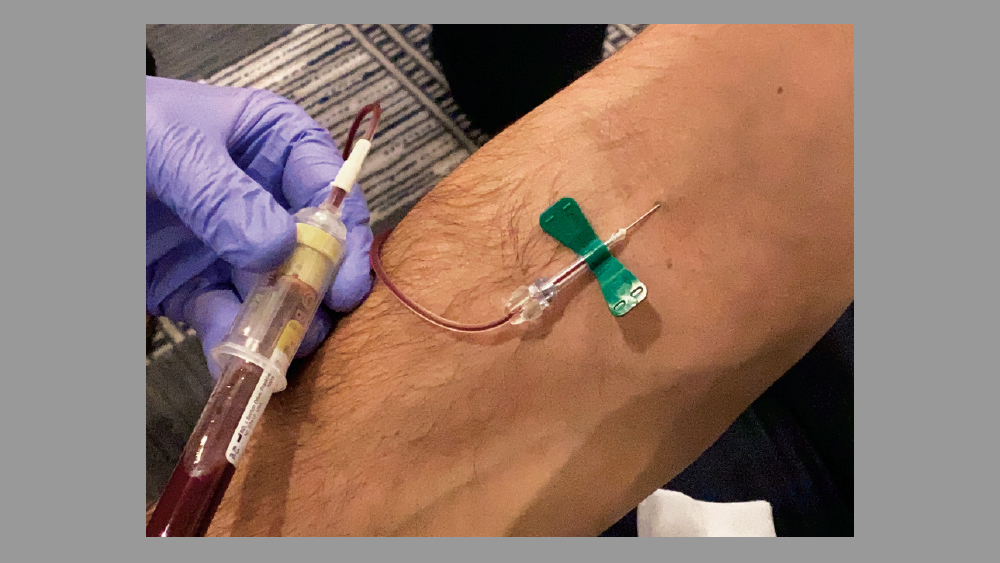
Figure 7: Alternative venipuncture technique: A butterfly needle is used to obtain the blood sample.
b. Blood Sample Centrifuge
When using a plain collection tube (without anticoagulant), the centrifuge process activates the coagulation process as soon as the blood comes into contact with the tube walls. Therefore, it is imperative that there is no extended delay between obtaining the blood sample and the start of the centrifuge process. If an extended delay occurs, the fibrin clot will be less distinct. Ideally, the centrifuge should spin at approximately 2,700–3,000 rpm for about 10–12 minutes.
c. Blood Concentrate
Two different intrinsic processes result from the high-speed centrifuging of a blood sample: blood coagulation and separation of the blood elements. The centrifugal force separates cells of different densities, which results in three layers of blood product: The top layer consists of an acellular supernatant platelet-poor plasma (PPP), the platelet fibrin clot forms the middle layer, and the bottom layer is composed of concentrated red blood cells. The top layer (PPP), which contains a small percentage of platelets, may be discarded or used to hydrate the particulate bone. The middle layer (PRF clot) may be used as a membrane or integrated with the particulate bone. The bottom layer (red blood cells) is discarded. (See Table 2 and Figs. 8–13.)
Table 2: Single-Spin Platelet-Rich Fibrin Protocol
Step 1: Immediately place blood collection tube in centrifuge for 10–12 minutes at approximately 2,700–3,000 rpm. Make sure the centrifuge is balanced with an even number of tubes and an equal volume of liquid.
Step 2: Carefully remove the blood sample tube from the centrifuge. Three distinct layers will be present:
- Top layer: Platelet-poor plasma (PPP)
- Middle layer: Platelet-rich fibrin (PRF)
- Bottom layer: Red blood cells (RBCs)
Step 3: Remove the rubber top from the blood sample tube. Using a sterile 5 ml syringe with a blunted needle, draw off the yellow liquid top later (PPP). This may be discarded or added to the particulate graft material.
Step 4: Using sterile college pliers (pick-ups), remove the fibrin clot (PRF) from the center of the tube. If coagulated RBCs are attached to the clot, they may be removed with scissors and discarded.
Step 5: PRF processing:
- Membrane: Place the fibrin clot into a PRF box or biocompress instrument to compress the clot. This will result in an approximately 1-mm-thin membrane. The PRF membrane is then placed over the graft site membrane, serving as a double membrane between the primary membrane (e.g., collagen) and the soft-tissue flap.
- Graft incorporation: The PRF clot may be cut into small pieces and added to the graft. In addition, the PPP may be added to the graft. In addition, the PPP may be added to the graft if more hydration is required, as the PPP does contain a low concentration of platelets.

Figure 8: For the PRF preparation technique, a sample is placed in the centrifuge, which is capable of reaching a speed of 3,000 rpm.

Figure 9: After 10–12 minutes of centrifuge spinning, three distinct layers are present: acellular platelet-poor plasma (PPP) on top, the PRF clot in the middle, and red blood cells (RBCs) on the bottom.
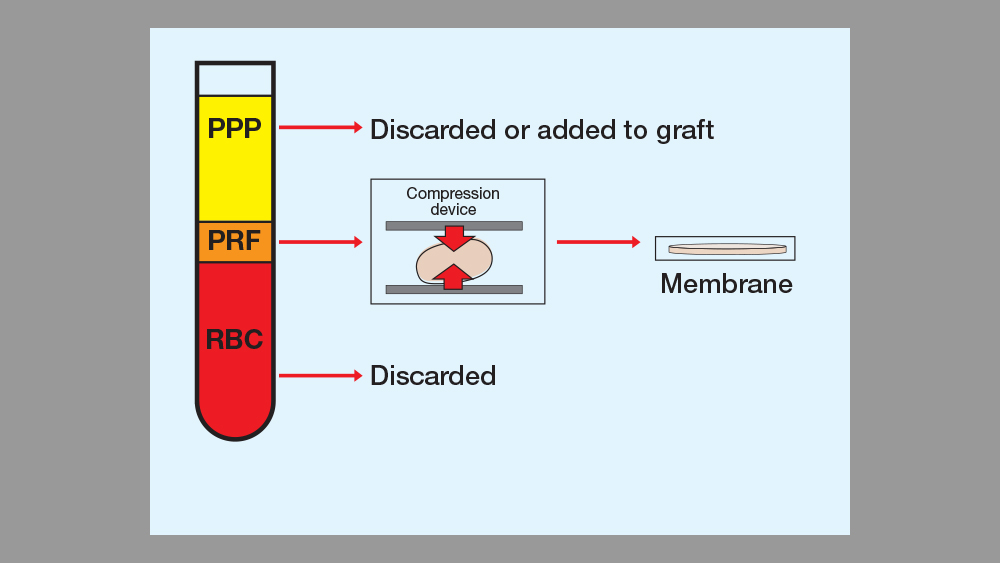
Figure 10: PRF processing: The centrifugal force separates cells of different densities, which results in three layers of blood product available for processing.
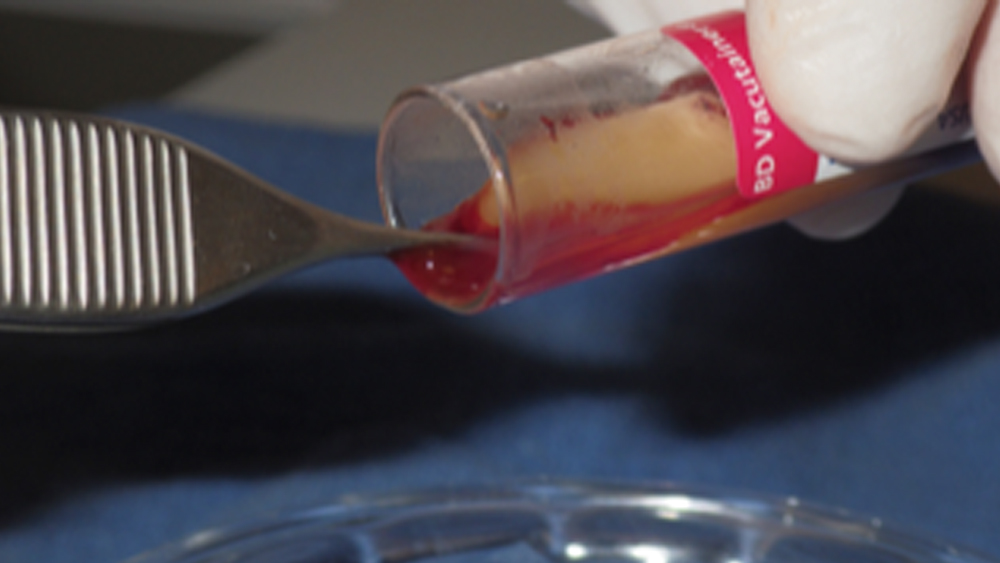
Figure 11: The fibrin clot is removed.
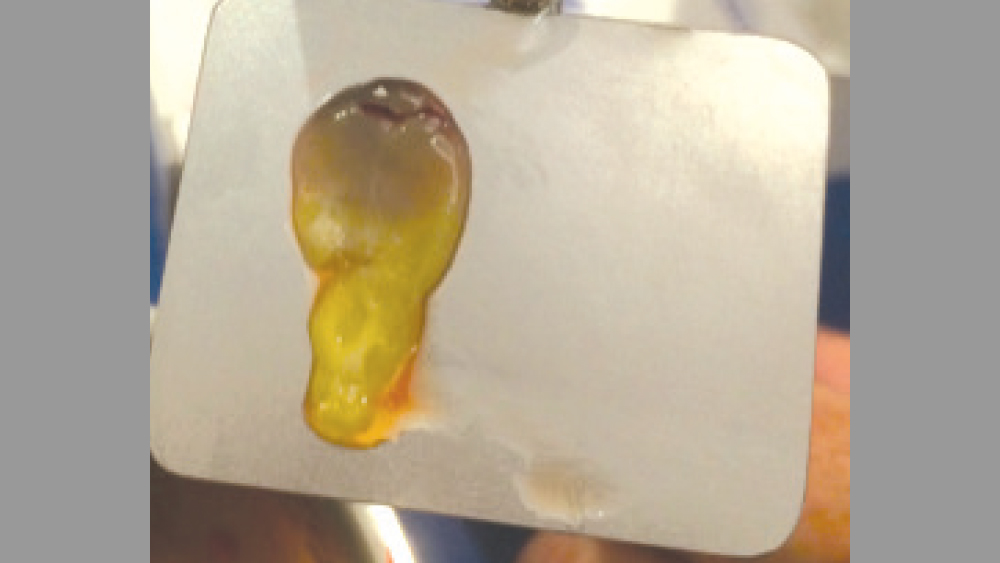
Figure 12: Use of a biocompress for a membrane.
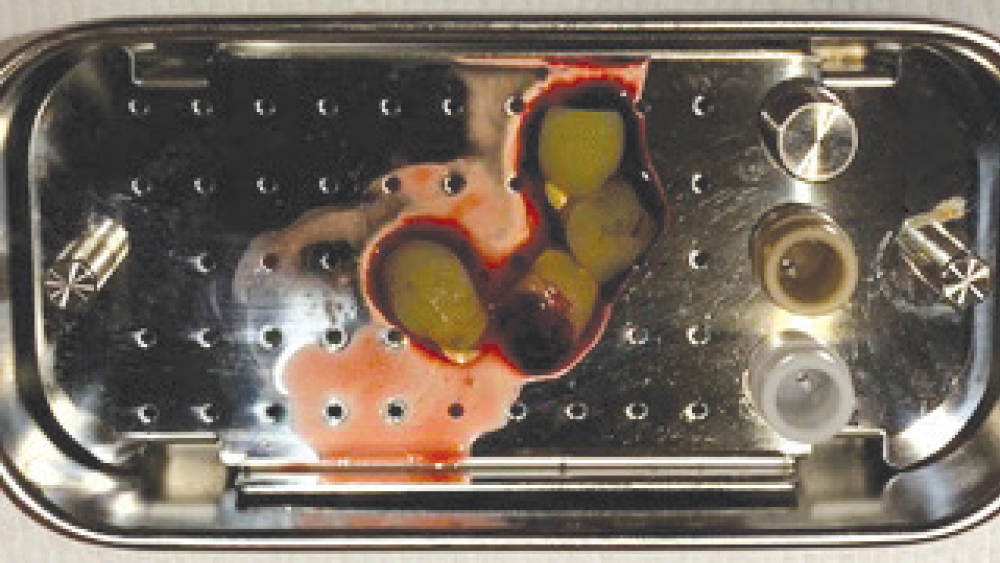
Figure 13: Fibrin clots placed in PRF box.
CLINICAL USES OF PLATELET-RICH FIBRIN
Platelet-rich fibrin has been used in many oral implant procedures, ranging from guided bone regeneration to procedures requiring a hemostatic agent (Figs. 14–18).
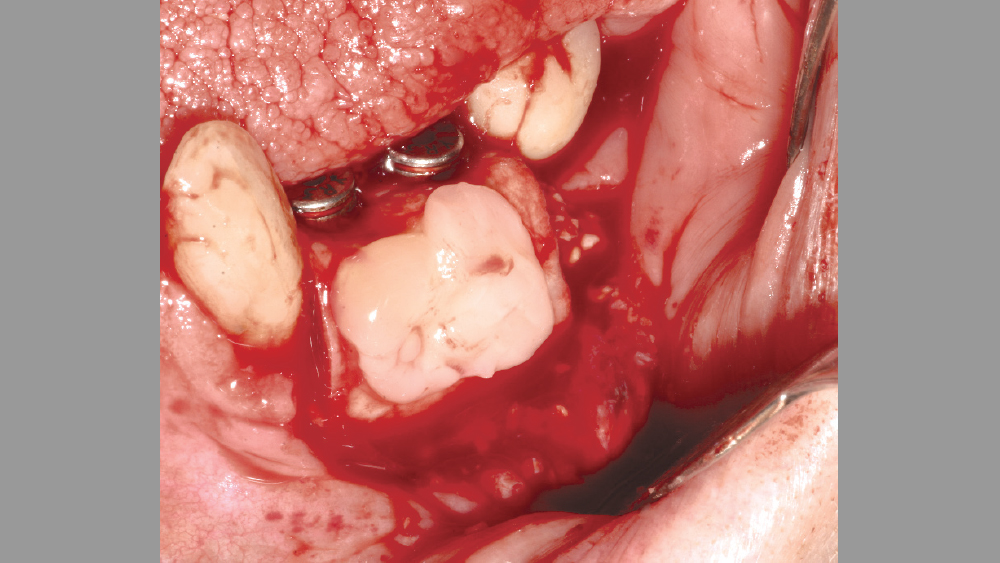
Figure 14: PRF used as a membrane after implant placement.
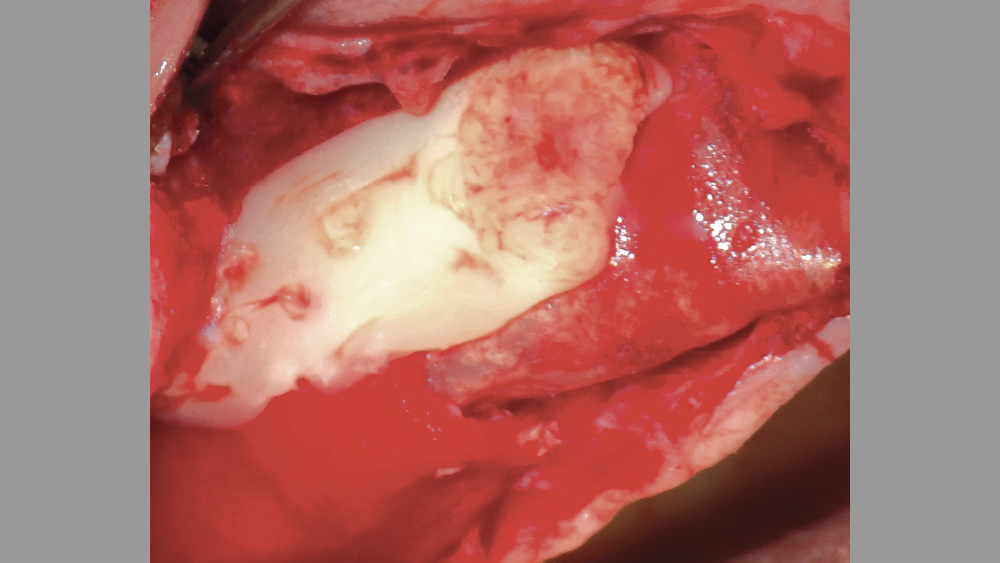
Figure 15: PRF used as a membrane over a lateral wall sinus augmentation.

Figure 16: PRF may be added to hydrate the graft material.
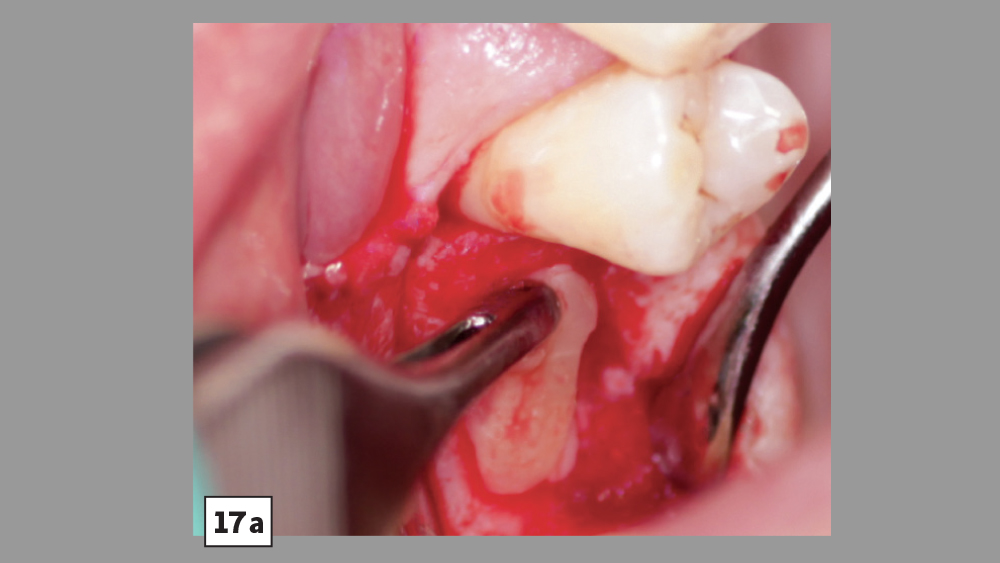
Figures 17a, 17b: PRF may be used in conjunction with crestal-approach sinus augmentation procedures.
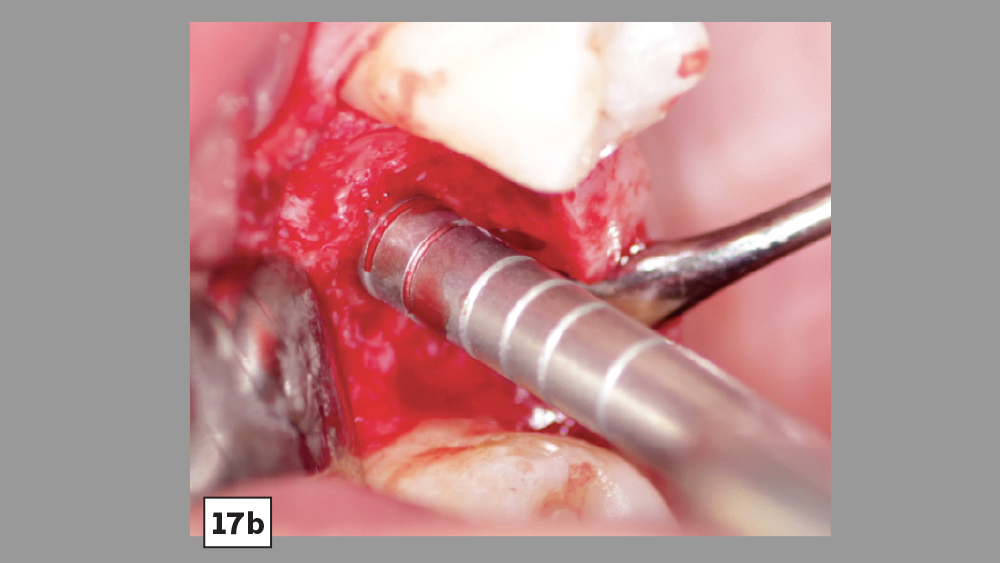
Figures 17a, 17b: PRF may be used in conjunction with crestal-approach sinus augmentation procedures.

Figure 18: A membrane may be hydrated in PPP or PRF.
Guided bone regeneration: With bone augmentation procedures, PRF may be used as either a membrane or added to the particulate bone grafting material. Studies have shown that PRF is advantageous in healing during regenerative procedures either as a membrane or when added to particulate bone.11 Because the PRF membrane resorbs rather quickly (approximately seven days), it is not the most ideal membrane to be used to prevent soft-tissue invasion. Therefore, the PRF membrane is commonly placed over the primary membrane (e.g., collagen) to aid in hard- and soft-tissue healing.
Sinus augmentation: During sinus augmentation procedures, PRF is often used with the graft material, as a second membrane over the lateral wall, or as a membrane during crestal approaches. Choukroun et al. showed that when PRF was added to freeze-dried bone allograft, there was a reduction in healing time prior to implant placement.12 Diss et al. showed that PRF used with the osteotome crestal approach for sinus augmentation procedures resulted in faster healing and sufficient torque values when implants were placed in areas with reduced bone height.13
Socket grafting: Many studies have evaluated the use of PRF after extraction as a socket membrane, sole socket filling material, or the addition to particulate socket grafting procedures. Most studies show positive healing effects when PRF is used in combination with socket graft procedures.14
Periodontal defects: The use of PRF in conjunction with periodontal and peri-implant disease procedures are well documented. Chang et al. reported that PRF is an effective modality in the treatment of infrabony periodontal defects.15
Hemostasis: PRF is an excellent hemostatic agent when used in procedures that may result in excess postoperative bleeding. PRF has been shown to exhibit very good antihemorrhagic properties, along with increased tissue healing, wound closure, and decreased postoperative pain. PRF membranes can be placed over the surgical site, or the surgical site can be lavaged with the material to decrease bleeding.16
CONCLUSION
In the field of oral implantology, clinicians are always looking for ways to provide faster and more predictable hard- and soft-tissue healing. The most commonly used blood concentrate, platelet-rich fibrin, has been shown to be advantageous in a full array of implant procedures. Whether using PRF as a membrane or integrating it into the particulate bone graft, a favorable physiologic support system is achieved, which allows for an enhanced and more predictable healing process. This article has summarized a simple, clinically proven protocol for the use of blood concentrates for bone regeneration procedures that may be easily integrated into the general dentist’s practice.
Available CE Course
References
- ^Froum SJ, Wallace SS, Tarnow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodontics Restorative Dent. 2002 Feb;22(1):45-53.
- ^Alissa R, Esposito M, Horner K, Oliver R. The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. Eur J Oral Implantol. 2010 Summer;3(2):121-34.
- ^Kiran NK, Mukunda KS, Tilak Raj TN. Platelet concentrates: a promising innovation in dentistry. J Dent Sci Res. 2011;2:50-61.
- ^Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998 Jun;85(6):638-46.
- ^Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101(3):e51-5.
- ^Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101(3):e56-60.
- ^Kang YH, Jeon SH, Park JY, Chung JH, Choung YH, Choung HW, Kim ES, Choung PH. Platelet-rich fibrin is a bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng Part A. 2011 Feb;17(3-4):349-59.
- ^Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101(3):e37-44.
- ^Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101(3):e45-50.
- ^Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016 Dec;20(9):2353-60.
- ^Montanari M, Callea M, Yavuz I, Maglione M. A new biological approach to guided bone and tissue regeneration. BMJ Case Rep. 2013 Apr 9;2013:bcr2012008240.
- ^Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101(3):299-303.
- ^Diss A, Dohan DM, Mouhyi J, Mahler P. Osteotome sinus floor elevation using Choukroun’s platelet-rich fibrin as grafting material: a 1-year prospective pilot study with microthreaded implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 May;105(5):572-9.
- ^Temmerman A, Vandessel J, Castro A, Jacobs R, Teughels W, Pinto N, Quirynen M. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: a split-mouth, randomized, controlled clinical trial. J Clin Periodontol. 2016 Nov;43(11):990-9.
- ^Chang YC, Zhao JH. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust Dental J. 2011 Dec;56(4):365-71.
- ^Sammartino G, Dohan Ehrenfest DM, Carile F, Tia M, Bucci P. Prevention of hemorrhagic complications after dental extractions into open heart surgery patients under anticoagulant therapy: the use of leukocyte- and platelet-rich fibrin. J Oral Implantol. 2011 Dec;37(6):681-90.



