Product Spotlight: Penguin RFA® Determining Implant Stability with Objective Measurements
Clinicians understand the importance of determining the stability, viability and health of each implant site prior to moving forward with prosthetic loading. Whether it is a determination of adequate primary stability to support immediate loading, or evaluation of the level of osseointegration prior to fabrication of the final restoration, successful treatment can often depend on the practitioner’s ability to make an accurate and objective assessment to support the clinical decision.
Successful treatment can often depend on the practitioner’s ability to make an accurate and objective assessment to support the clinical decision.
Resonance frequency analysis (RFA) is a simple and noninvasive method of testing implant stability. The technique provides information about the state of the implant-bone interface. Measurements may be taken at the time of implant placement to obtain a baseline value, as well as during follow-up treatment to monitor osseointegration and decide when to load.
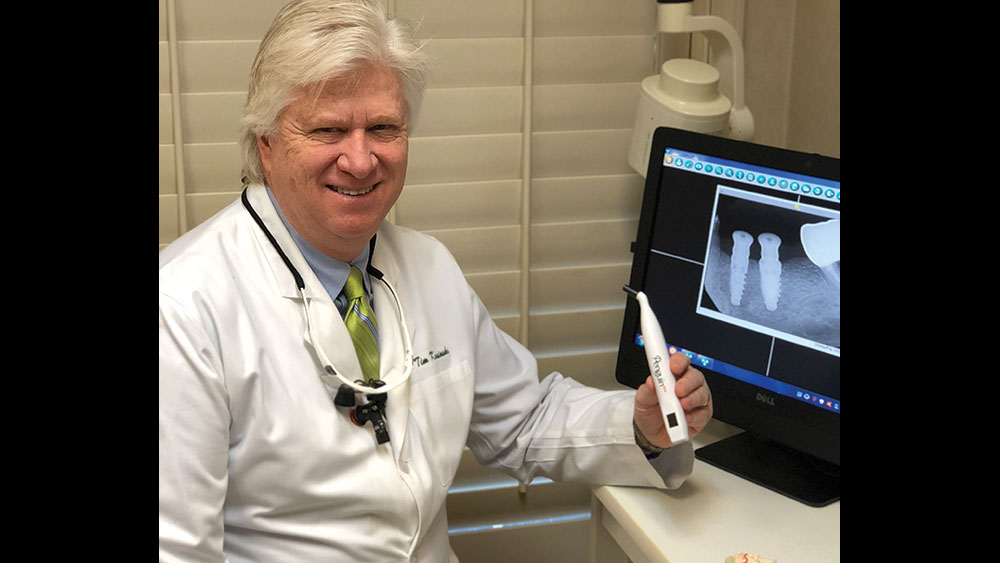
RFA TECHNIQUE
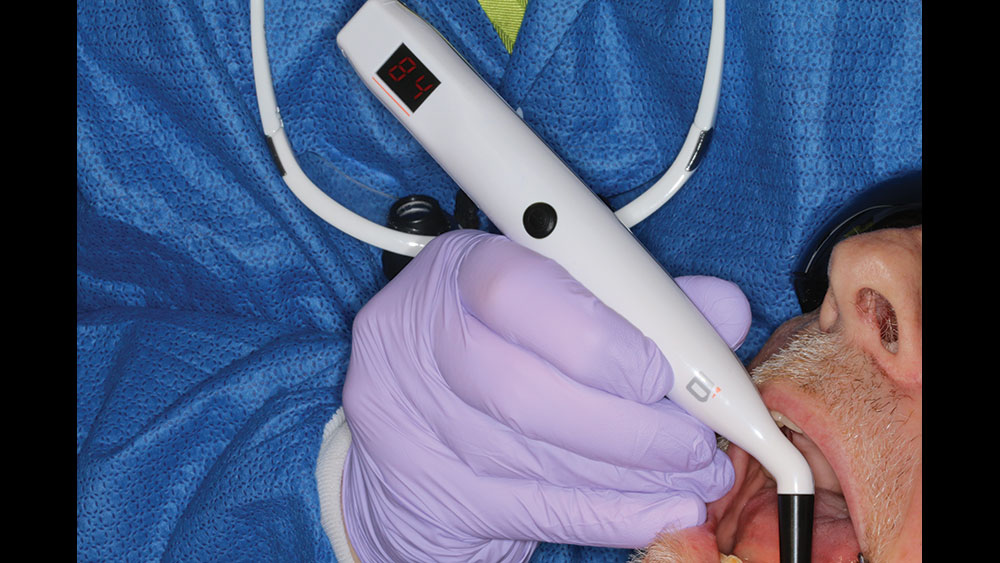
The Penguin RFA® (Glidewell Direct; Irvine, Calif.) provides an accurate assessment of implant stability at any step in the treatment process. This instrument is used together with a MulTipeg™, which is a transducer that is attached to the implant and stimulated over a range of frequencies by electromagnetic waves. The stability of the implant is presented as an implant stability quotient (ISQ), with a range from 1 (lowest stability) to 99 (highest stability) ISQ units. RFA measurements reflect the micromobility of dental implants, which is influenced by the bone density at the implant site, the implant placement technique, implant design, healing time, bone remodeling and regeneration, and exposed implant height above the alveolar crest.
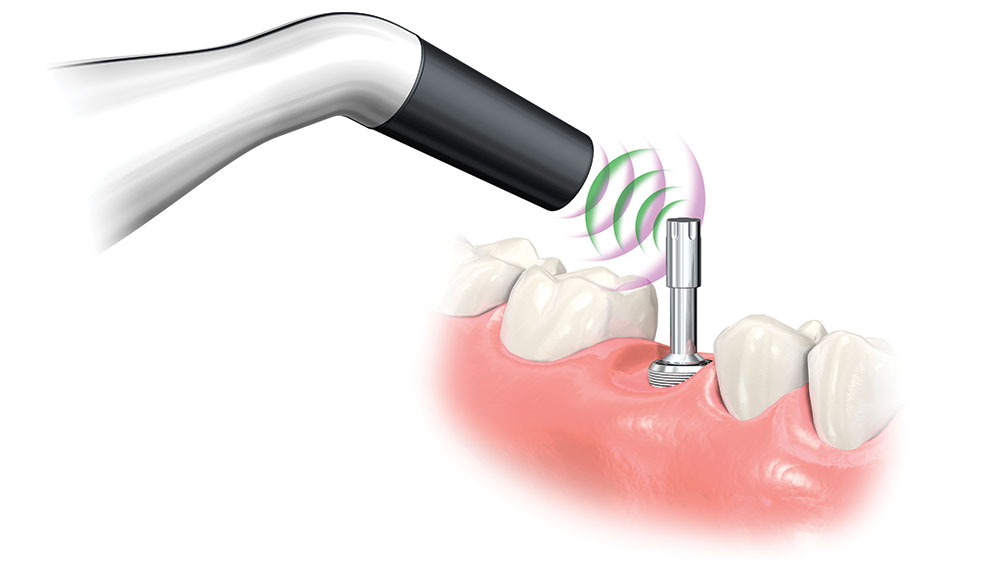
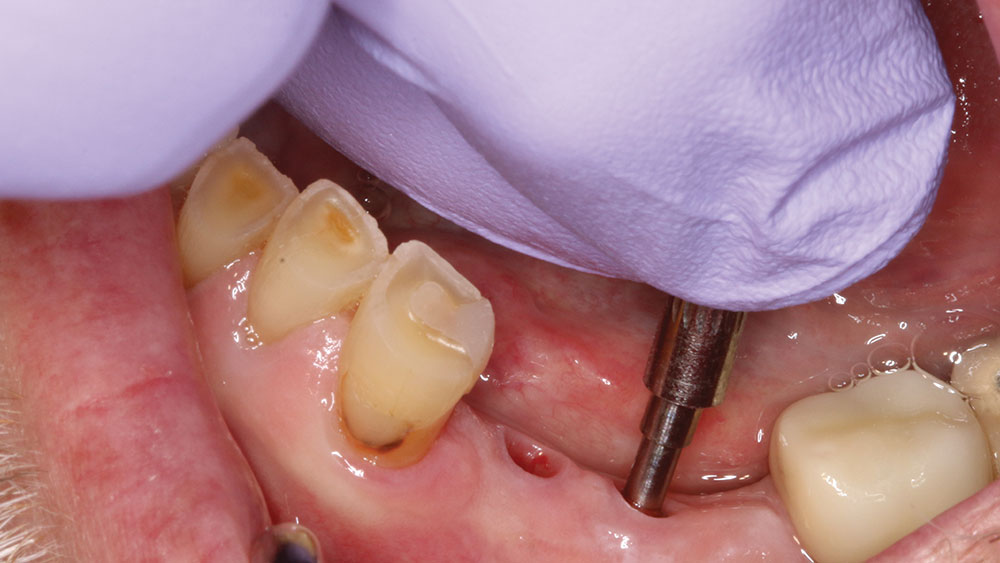
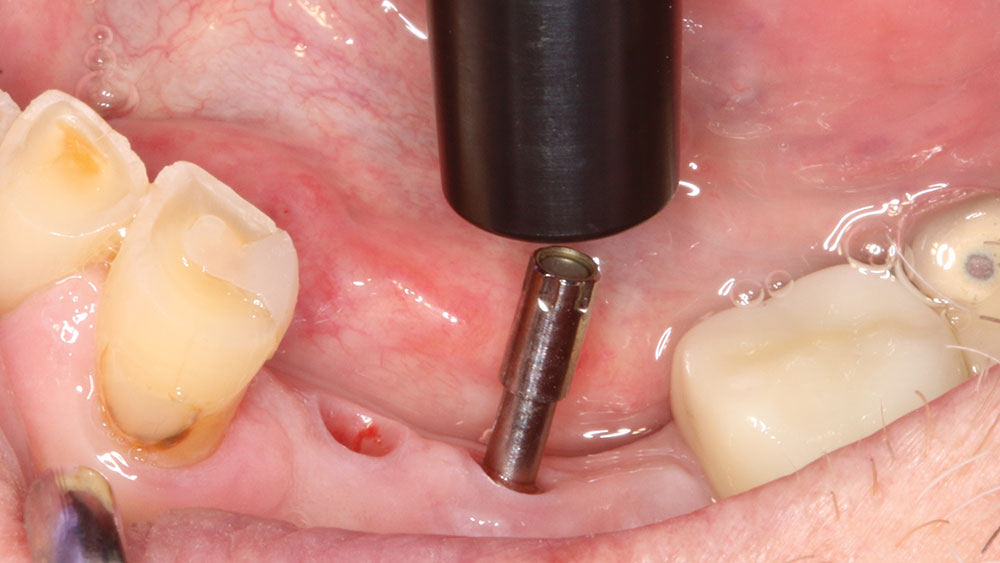
To take the stability measurement, connect the MulTipeg to the implant and aim the Penguin RFA instrument toward the magnet at the top of the peg. The peg is stimulated by magnetic pulses and vibrates due to the stiffness in the contact area between the bone and implant interface.
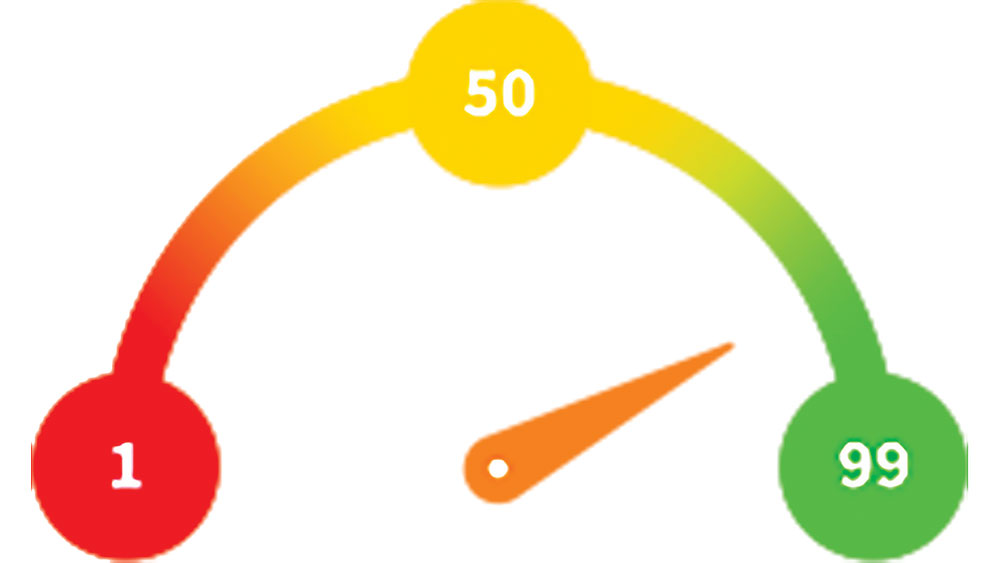
The stability of implants is indicated on an ISQ scale of 1–99. By taking a baseline value at implant placement and another before loading, the degree of osseointegration can be determined.
Generally, values above 70 ISQ indicate a very stable implant with low micromobility that can withstand normal functional forces in the mouth. This measurement can confirm that one-stage immediate loading is appropriate and predictable. Measurements of less than 60 ISQ indicate that more time should be allowed for integration prior to loading. Values in the range of 75 ISQ typically indicate the implant is ready for the final restoration. Because an implant can have different ISQ values depending on the position of the instrument, measurements are taken in several directions. In combination with clinical and radiographic evaluations, the technique is effective in validating implant site readiness for the restorative phase of implant treatment.
The technique is effective in validating implant site readiness for the restorative phase of implant treatment.
CASE REPORT
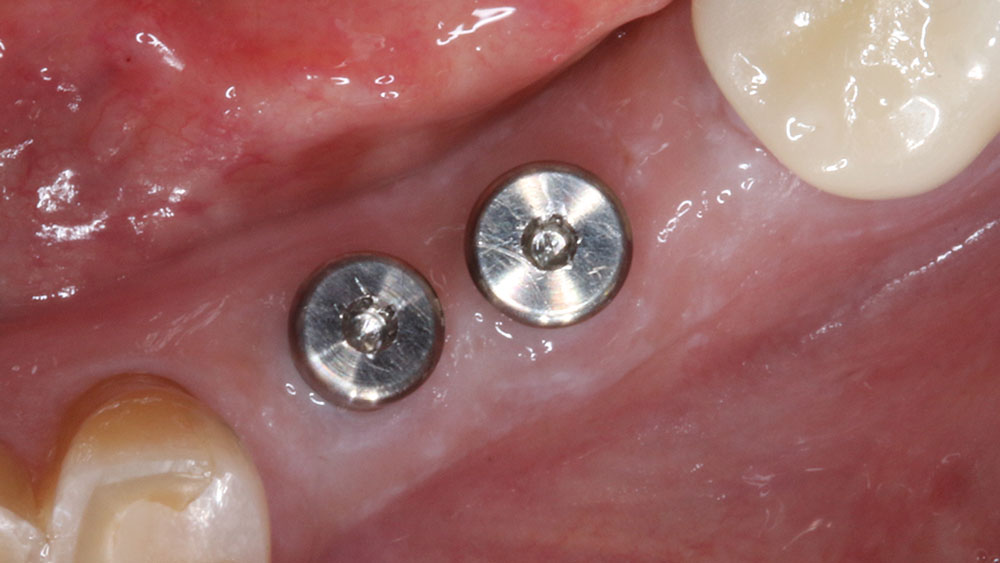
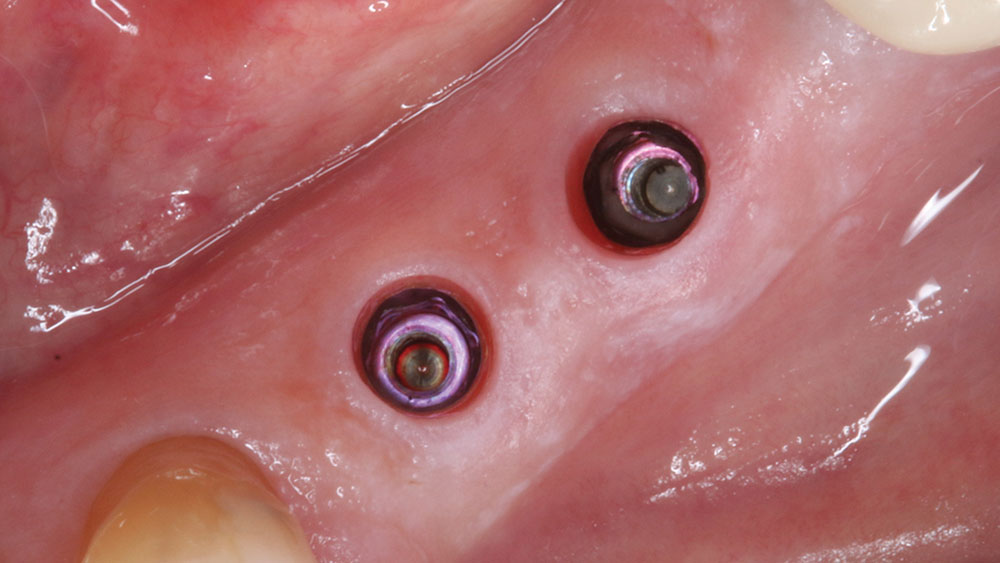
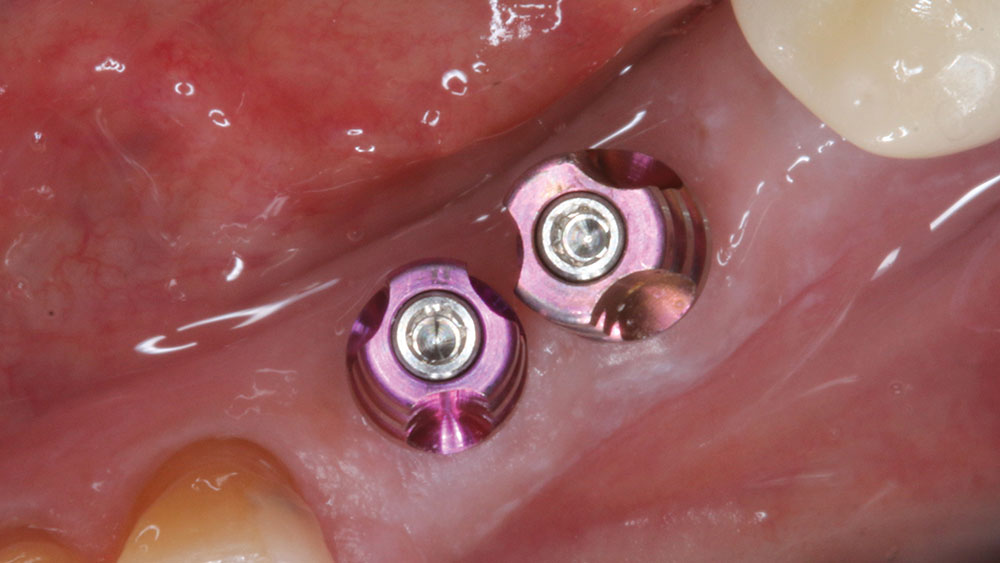
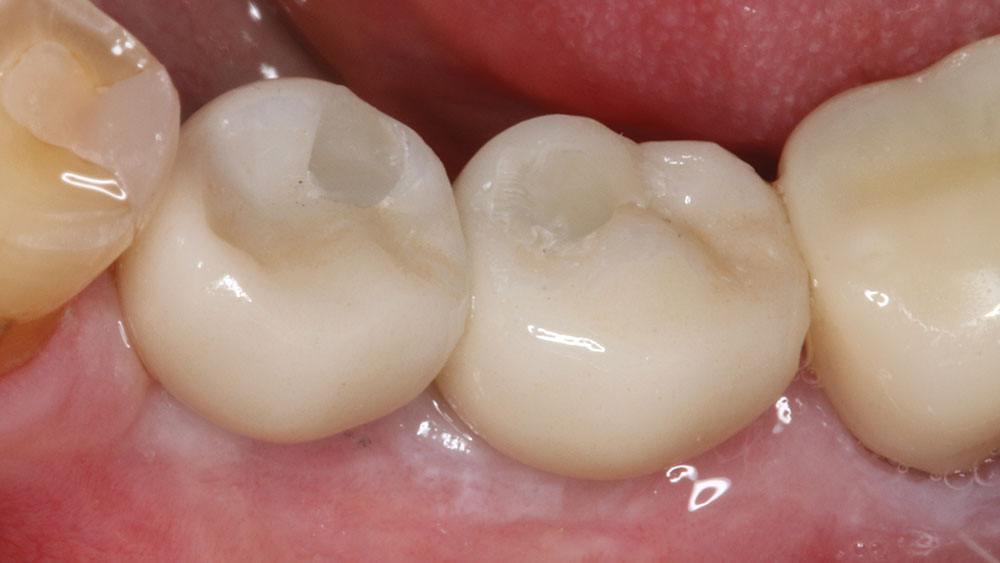
For implant therapy, I utilize the Hahn™ Tapered Implant (Glidewell Direct) due to its ability to achieve excellent primary stability and facilitate an ideal esthetic outcome, as well as its straightforward surgical protocol. I use the Penguin RFA to measure the stability of each implant prior to making my final impression, as illustrated on the next page. It’s a simple and cost-effective aid in clinical decision-making.
CONCLUSION
Practitioners are encouraged to test dental implants to determine stability at the time of placement and again prior to impressing. The Penguin RFA provides reliable, objective and repeatable measurements. The RFA technique supports decision-making and aids in determining the most effective path to maximize long-term, successful clinical outcomes.
Penguin RFA is a registered trademark of Integration Diagnostics Sweden AB.