Breakthrough 3D-Printed Denture Eliminates Over 99.9% of Tested Bacteria and Fungi
For decades, it has been recognized that biofilm build-up on removable dentures exposes patients to the risk of stomatitis, oral malodor, MRSA, pneumonia and other disease states. While adherence to a rigorous cleaning protocol, proper storage and professional maintenance have traditionally been the best defense against biofilm buildup, there is a new product that eliminates virtually all bacteria and fungi: the denture material itself.
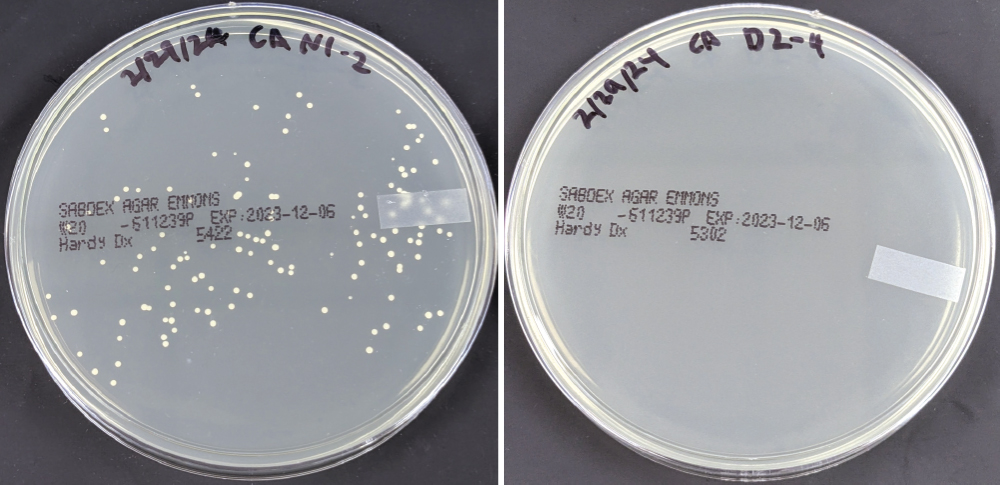
The 3D-printable denture resin, developed by Glidewell’s research & development group and made by Glidewell’s ISO-certified manufacturing division, Prismatik Dentalcraft, has been shown to reduce over 99.9% of the most common bacteria and fungi known to cause biofilm.*
Glidewell has completed a series of studies demonstrating the efficacy of this denture resin in dramatically reducing the presence of colonizing pathogens over six months of accelerated use — equivalent to 36 months of real-time use. A recently submitted paper, titled “Antimicrobial efficacy of a novel antimicrobial 3D-printed denture resin,” states that “a denture printed from a resin system with strong antimicrobial properties and compatibility with the oral environment may lead to cleaner, less odorous dentures and reduce the risk of oral inflammation and denture-associated infections.” The FDA recently cleared the antimicrobial properties of the resin, a first in the dental industry.
“We take pride as an organization in investing in and developing unique technologies to serve the dental community,” said Glidewell CEO Stephenie Goddard. “Rather than buy run-of-the-mill printable denture resins from a third party, we set out to design and obtain clearance for our own resin materials — challenging our R&D team to provide a compelling antimicrobial feature in the process.”
“We deliver thousands of dentures annually to dentists, and we see firsthand the negative effects of bacteria and fungi on the surface of the denture,” said Raj Malyala, vice president of materials R&D at Glidewell. “All species tested in our study are clinically relevant and constitute potential infection. Our research demonstrates that the denture disks with the antimicrobial compound led to a highly significant reduction against all tested microbes.”
The Glidewell denture resin formulation contains silver sodium hydrogen zirconium phosphate as the antimicrobial agent. In vitro studies demonstrated over 99.9% reduction coupled with long-term effectiveness against Streptococcus mutans, Streptococcus mitis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Candida albicans, Candida glabrata, Candida tropicalis, Methicillin-resistant Staphylococcus aureus (MRSA), and Vancomycin-resistant Enterococcus (VRE). These microorganisms have been shown to accumulate as biofilm on the surface of a printed denture, contributing to denture stomatitis. MRSA and VRE are superbugs known to cause infections in hospitals and intensive care units.
Currently, Glidewell’s denture resin with antimicrobial properties is used for all Simply Natural™ Digital Dentures, and dentists do not need to specifically request the resin when prescribing the product.
While the antimicrobial properties of the denture resin are the most innovative feature, Simply Natural Digital Dentures provide additional benefits for the patient. Available in 15 VITA Classical shades, the printed denture teeth are esthetic and highly resistant to staining. The denture, with a flexural strength of 95 ± 5 MPa, is more resistant to breakage than competitive materials. The flexural modulus of 2,900 ± 150 MPa is higher than competitive resins, leading to less distortion. Additionally, Glidewell provides a no-fault remake policy, one-year warranty, and five-day in-lab working time for Simply Natural Digital Dentures.
To learn more about Simply Natural Digital Dentures and additional denture solutions, visit glidewell.com/dentures.
*Testing for bactericidal and fungicidal effects was conducted under in vitro conditions. Long term test and antibiofilm testing was conducted on S. mutans and C. albicans. Antimicrobial contact tests were performed against Streptococcus mutans, Streptococcus mitis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Candida albicans, Candida glabrata, Candida tropicalis, MRSA (Methicillin-resistant Staphylococcus aureus), VRE (Vancomycin-resistant Enterococcus). Data on file.