3 Practical Steps You Can Use to Treat Sleep-Disordered Breathing Immediately

During the past 10 years, there has been a mountain of literature discussing the high incidence of sleep-disordered breathing (SDB) in our population, and how important it is for dentists to screen for this condition. However, for dentists, the diagnostics and treatment protocols can be complex and time-intensive. Once dentists screen and identify patients with risk factors for SDB (e.g., enlarged tonsils and tongue, habitual snoring and hypertension), the path to treatment with a mandibular advancement device is often slow and arduous. Patients must receive a sleep test, which requires a referral to a sleep physician. Then the sleep physician must see them, and because there are only about four thousand sleep physicians in the entire country, this is difficult. Once patients are seen by the sleep physician, they must coordinate sleep testing, and then obtain the results of the sleep test prior to a definitive diagnosis and treatment. It’s a clunky, onerous process that creates barriers to successful follow-through.
To overcome these difficulties for the dentist desiring to provide this much-needed care, the PMAD (provisional mandibular advancement device) treatment protocol has been developed. When a routine screening determines that patients snore or suffer from excessive sleepiness, dentists can address their patients’ immediate health needs by making a provisional diagnosis and providing provisional therapy. The PMAD protocol uses a straightforward, three-step process that avoids delays and simplifies the complexities of conventional treatment. Using this method can help prevent health problems that may occur from untreated SDB.
TREATMENT PROTOCOL
STEP 1: IDENTIFY AND QUALIFY PATIENTS FOR PMAD THERAPY
When patients are at risk for sleep-disordered breathing, dentists can address their immediate health needs with provisional mandibular advancement device therapy.
The most common breathing-related issue is the presence of snoring. During the clinical exam, associated findings include the presence of crowding in the posterior airway by the tongue or tonsils, or both, and a thick neck larger than 17 inches for men or 16 inches for women. Other related issues include daytime sleepiness, pauses in breathing observed by the patient’s significant other, and systemic issues, such as hypertension or being overweight.
The STOP-BANG Questionnaire, a screening tool available from Glidewell, is a great resource because it can be given to every patient. For example, when the staff is updating a patient’s health history during a follow-up visit, the STOP-BANG Questionnaire can be provided. If the history and exam show that a patient is at risk for sleep-disordered breathing, PMAD therapy may be the best option to deliver immediate relief.

The STOP-BANG Questionnaire is an easy and reliable tool for predicting risk for obstructive sleep apnea.
STEP 2: INFORMED CONSENT
In the conventional treatment model, the biggest problem at this stage is accessibility of the required testing. The time between identification of the risk factors and obtaining the definitive diagnosis and rendering definitive treatment could be substantial. Sleep apnea is a medical condition that requires a diagnosis and management by a physician. However, to improve speed and access to treatment, dentists may provide at-risk patients with a provisional diagnosis. Dentists should document this disclosure with an informed consent document in the patient’s file before initiating treatment. Glidewell provides a medicolegal document that dentists may use in their practices. The informed consent document highlights the importance of providing provisional therapy while the patient awaits a definitive diagnosis, which often takes several months. This document is part of the Glidewell Sleep Solutions Kit and may be downloaded for free at glidewell.com/sleep-solutions-kit.
It’s important to have conversations about provisional therapy with patients. For example, I may say to my patients: “Because we care about your health, we screen all patients for obstructive sleep apnea, which is a significant health issue. According to our screening, you have several risk factors and will most likely require treatment.” I’d proceed by reviewing my findings, and by providing a written summary of the patient’s evaluation to help them when they speak with their physician. Then I’d explain provisional treatment: “Between now and the time that we get this definitive diagnosis completed, because we recognize there is a number of steps involved and we want to take advantage of your coverage whenever possible, we’re going to fabricate a provisional device, which will help introduce you to treatment options.”
STEP 3: PROVIDE PROVISIONAL TREATMENT
When choosing provisional therapy, dentists should select a device that fits well, is comfortable to use and will provide improvement. Some patients may wonder if an over-the-counter, boil-and-bite appliance may be appropriate. These devices are a bad idea because, in my experience, when people use a boil-and-bite appliance and don’t respond to treatment (i.e., don’t have a positive or beneficial experience), they consequently believe all oral appliances don’t work for them, and they don’t want to pursue treatment with a customized, dentist-prescribed device. I do not recommend using temporary, over-the-counter devices.
Provisional therapy must be comfortable and adjustable, and it should mimic the final device. The cost and ease at which you can have it made must also be considered. The Silent Nite® Sleep Appliance (Glidewell; Newport Beach, Calif.) is well suited for provisional therapy because it is clinically effective, it includes a fast turnaround time, with only three days in the lab, and it fits the criteria to ensure patients have an optimal first experience with oral appliance therapy.
To help make choosing the right appliance easier, dentists may use the comparison matrix from Glidewell. It’s a simple chart that illustrates the features and benefits of some of the most widely used sleep appliances in the field. After selecting the appliance that fits the patient’s clinical situation, all that’s needed is upper and lower impressions and a bite registration. We start everybody at a setting that is almost universal: an edge-to-edge bite registration. I typically say to patients, “Bite the edges of your front teeth together like you’re biting thread.” Almost everybody can find that position, and that position almost never puts strain on the jaw.
Delivering the case is straightforward, and the appliance can be titrated to provide a comfortable position that reduces symptoms.
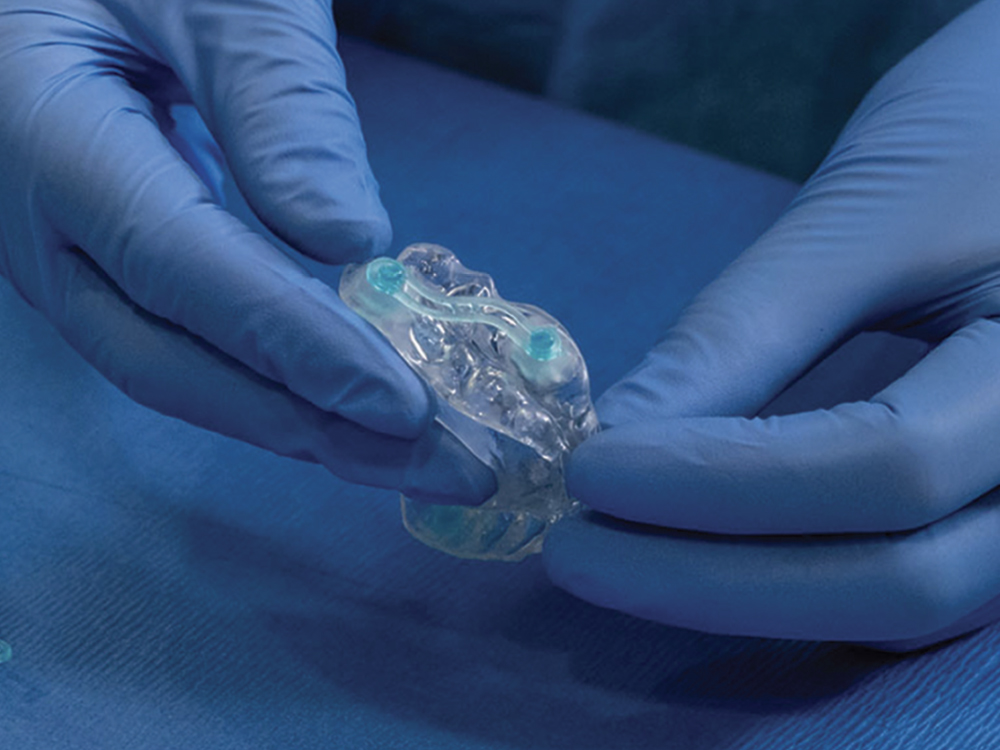
FDA-cleared for the treatment of snoring and obstructive sleep apnea, the Silent Nite Sleep Appliance is well suited for provisional therapy.

Available as a free resource from Glidewell, the Sleep Solutions Kit includes the STOP-BANG Questionnaire, medicolegal informed consent document and sleep appliance comparison matrix. To download these documents for your practice, visit glidewell.com/sleep-solutions-kit.

PAYMENT FOR PMAD THERAPY
Dental insurance does not pay for sleep appliances. Medical insurance can be complex and time-intensive, and will not pay unless a physician provides the definitive diagnosis. Therefore, the patient is charged directly for the provisional appliance and related chairside services. From there, follow-up visits may be scheduled.
SLEEP RECALL
It’s important to have an established recall schedule. If a person is identified as being at risk for sleep-disordered breathing and provisional therapy is delivered, the patient needs to be put on a different recall: a sleep recall. For this schedule, four weeks after provisional therapy is provided, the patient is seen again, either via a telehealth visit or in-person visit. This visit helps ensure patients don’t fall through the cracks.
When creating a new treatment pathway algorithm, it’s appropriate to schedule one-month recalls until stability is obtained or more definitive diagnostics are completed. This gives the office the opportunity to stay more engaged with the patient and the disorder.
CONCLUSION
Every dental practice should provide oral devices for the treatment of sleep-disordered breathing, and any dental practice can very easily utilize the PMAD protocol. It’s a simple, health-conscious approach that solves the time to treatment problem, and most importantly, it provides immediate treatment for a potentially life-threatening medical condition. General dental practices can get started by employing several resources, including the STOP-BANG Questionnaire, informed consent medicolegal document and sleep appliance comparison matrix, which helps dentists in selecting an FDA-cleared medical device that is best suited for the individual patient’s needs. Once the appliance is delivered, and the patient uses the device as prescribed and titrated by the dentist, it is expected that the patient will experience better health and improved quality of life.



