Selecting Grafting Materials Based on the Anatomical Defect
One of the most confusing aspects of hard-tissue grafting revolves around what material to use and where. This confusion arises due to the wide variety of grafting materials available to practitioners today. The goal of this article is to help you understand the process of successful bone growth, the types of grafting materials and the basic principles of their use.
The goal of bone grafting is to regenerate bone within the defect. In order to regenerate bone, certain conditions must be met:
- The recipient site must have an adequate blood supply
- The graft material must have both organic and inorganic components to provide a framework for new bone growth
- The graft material and the recipient site must be stable
- Adequate time must be given to completely replace the graft material with native bone
GRAFT MATERIALS
There are four main categories of graft materials available on the market for dental use: allografts, alloplasts, autografts and xenografts. Each material has advantages and disadvantages that should be considered when grafting hard tissue defects. In this article, I discuss two of the most commonly used grafting materials: allografts and alloplasts.
Allografts
Allograft materials can be obtained from either a compatible living donor or from cadaveric bone sources.1 On average, allograft particulates require 3–4 months of healing time to turn over. Particulate is the most commonly used form of this material. It comes in either mineralized or demineralized forms and either cortical, cancellous or blended varieties.
Mineralized bone contains both organic and inorganic components, while demineralized bone does not. The demineralized bone is treated during production to remove the inorganic component. Most of these products then have a carrier composed of methyl cellulose or analogous compounds to bind them together. In my experience, these products tend to “wash out” due to their consistency, unless the binder is manufactured to resist this dissolution.
On its own, cortical particulate keeps its volume excellently, providing a storehouse of minerals for new bone growth. Cancellous particulate does not resist compression as well as cortical particulate, but it does have a high percentage of collagen — which makes it great for cellular and vascular ingrowth. Because the particulates have contrasting properties, the literature has shown that a blend of these two types of bone is ideal.2 One example of blended material is the Newport Biologics™ Mineralized Cortico/Cancellous Allograft Blend (Glidewell Direct; Irvine, Calif.).
Alloplasts
This diverse category of products is made up of mineralized materials derived from nature or developed in a manufacturing process. Many of these products are then combined with collagen, which results in a matrix that is ideal for bone growth and a stable material that resists migration. On average, alloplasts turn over between 3–6 months, but some take much longer.
Practitioners must be careful about what materials they choose because not all alloplasts are alike. Some products are manufactured by exposing minerals to very high heat, which results in a bio-ceramic that is very difficult or impossible for the body to resorb. I prefer to use the Newport Biologics Bone Graft Putty and OsteoGen® Plugs (Glidewell Direct) for sites that require alloplast materials.
Now that we’ve covered the basics, let’s look at a few different scenarios and decide how to choose the best grafting material. Before starting, you should always remember that regenerating bone for infrabony defects is very predictable, but it becomes harder as the defect gets larger and as you move outside the bony envelope. Once outside the confines of the bone architecture, height is more difficult to manage than width under most circumstances.
SOCKET PRESERVATION
In most cases, an infrabony defect will have tremendous osteogenic potential, due to the stable native bone surrounding the graft material. This makes the choice of material less important. Essentially, all materials will work well, but they differ in their costs and handling properties. Ultimately, it becomes a choice of what material the practitioner is comfortable with. I recommend using Newport Biologics allograft and alloplast particulate, RAPTOS® particulate (Glidewell Direct) and OsteoGen Plugs.
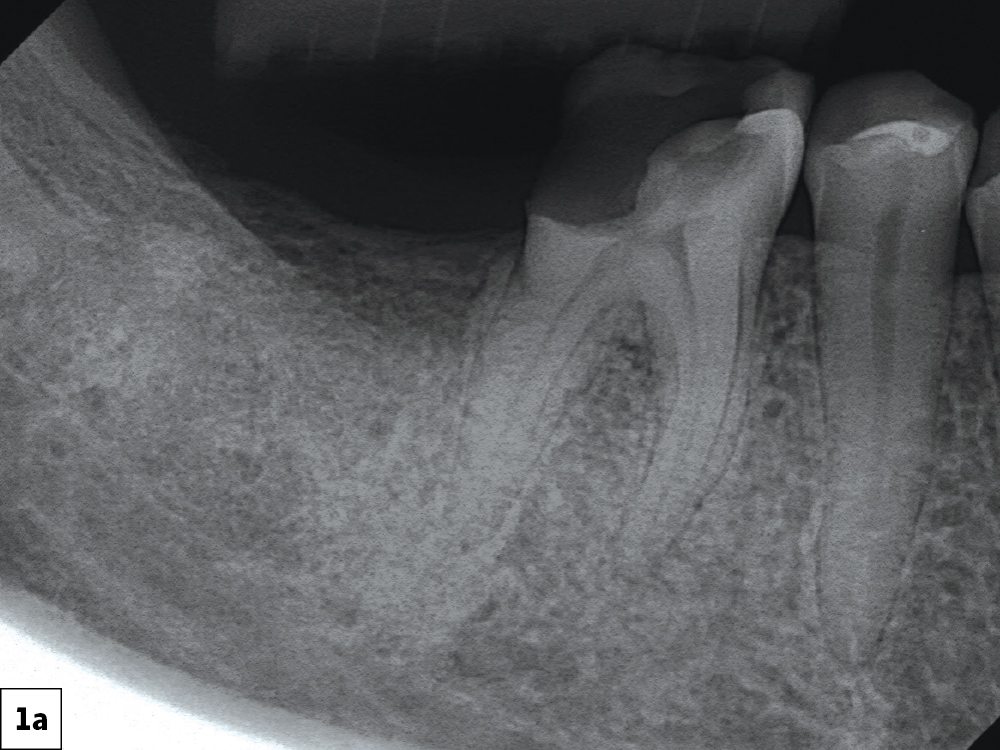
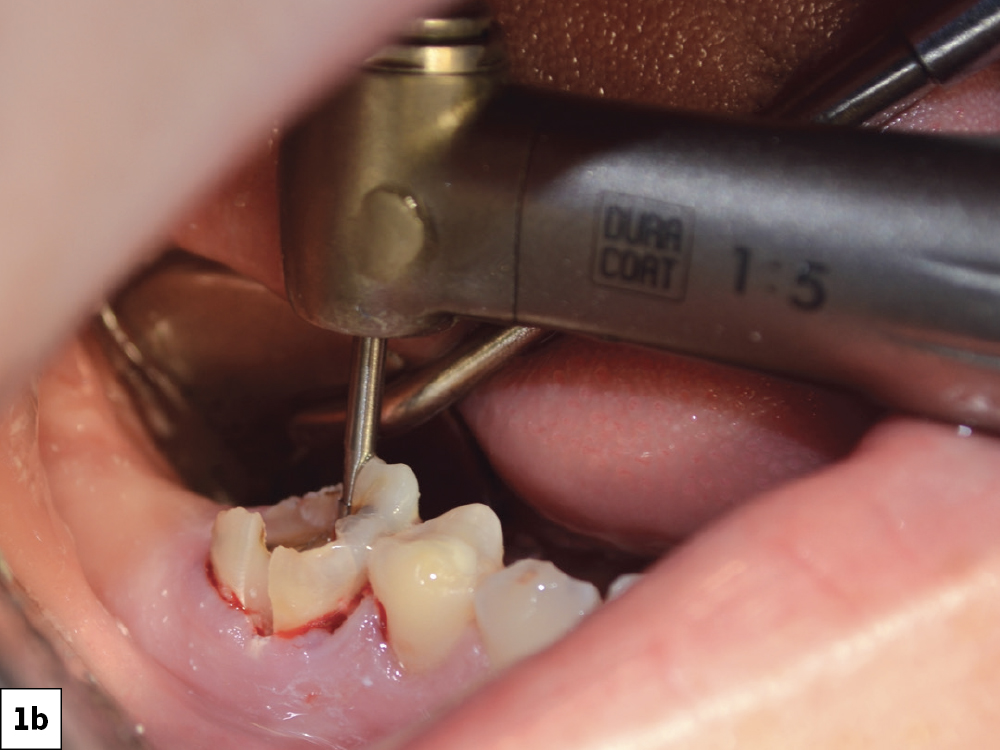
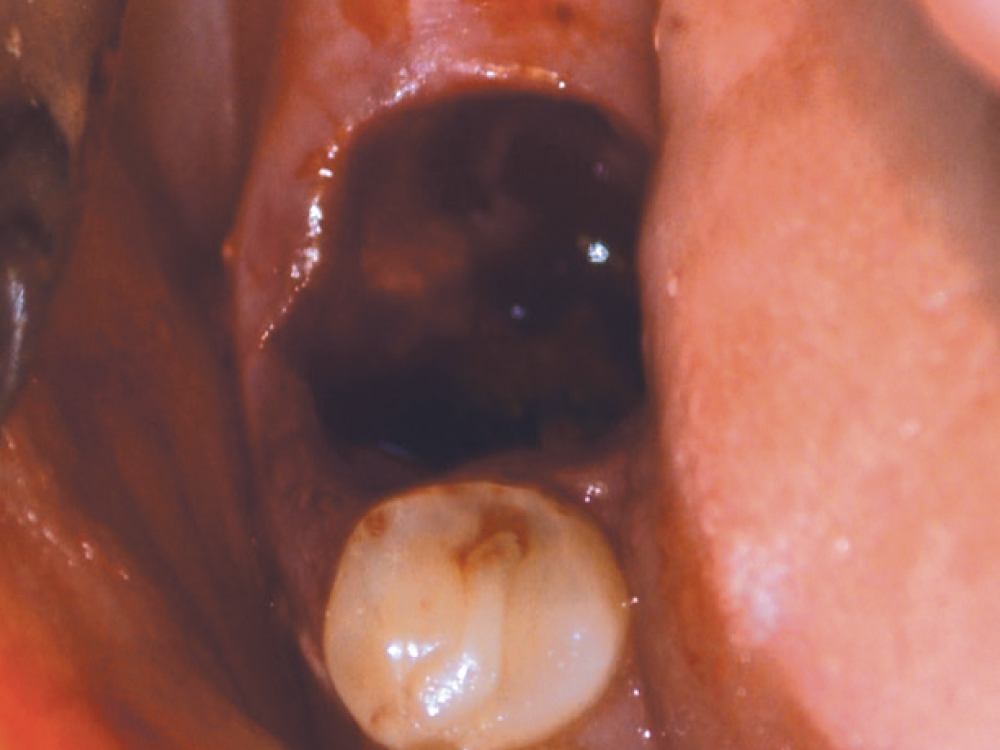
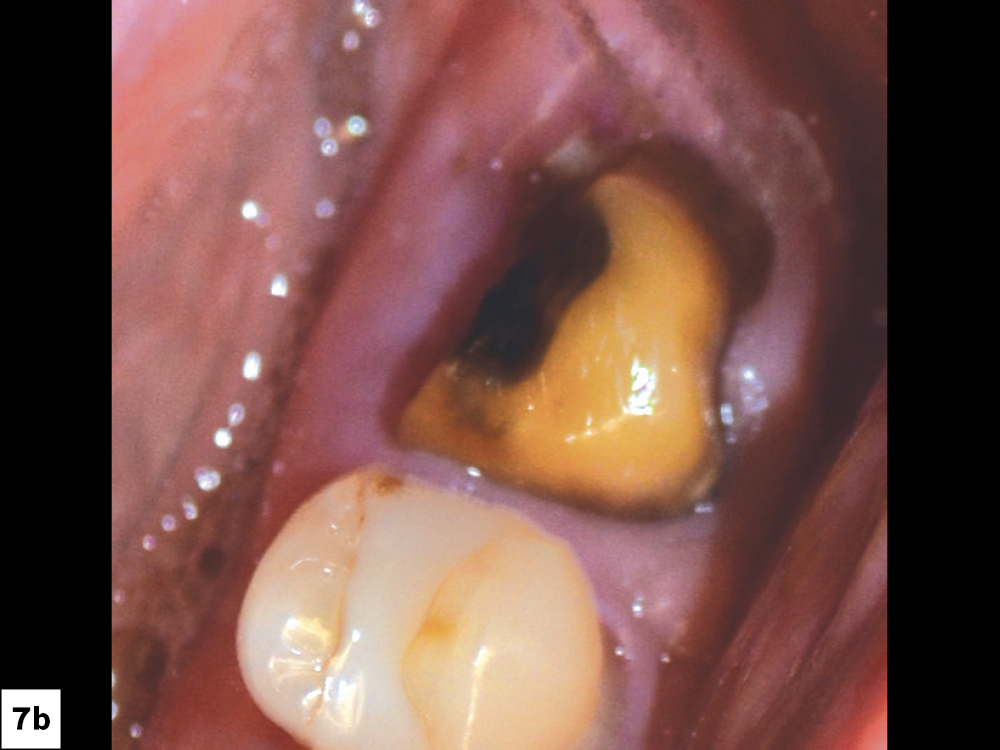
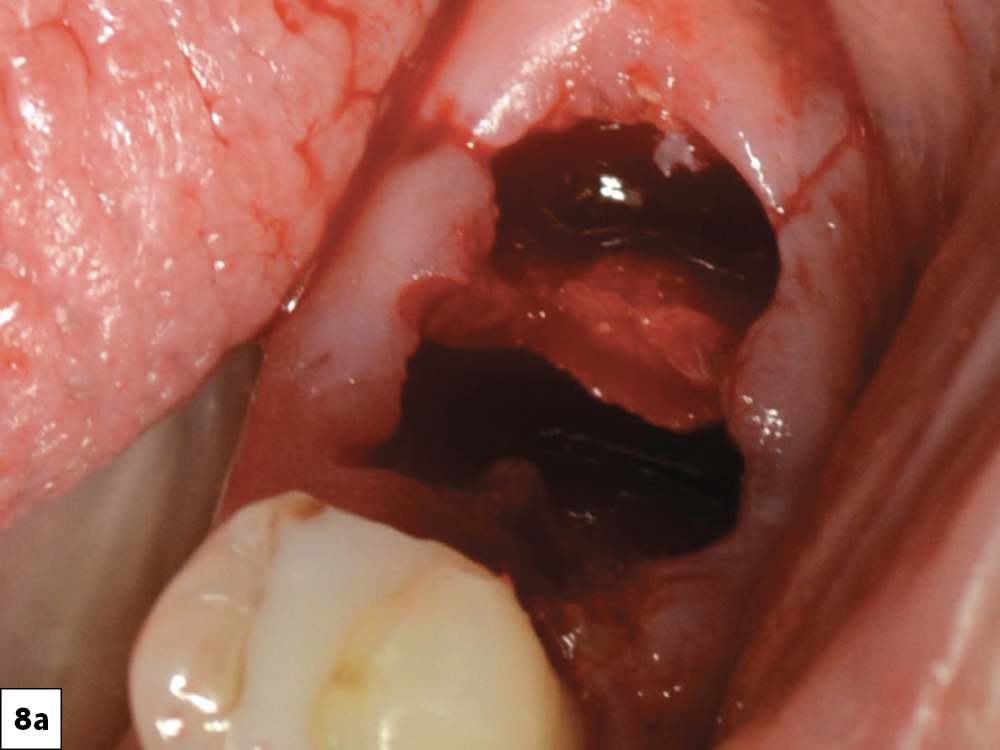
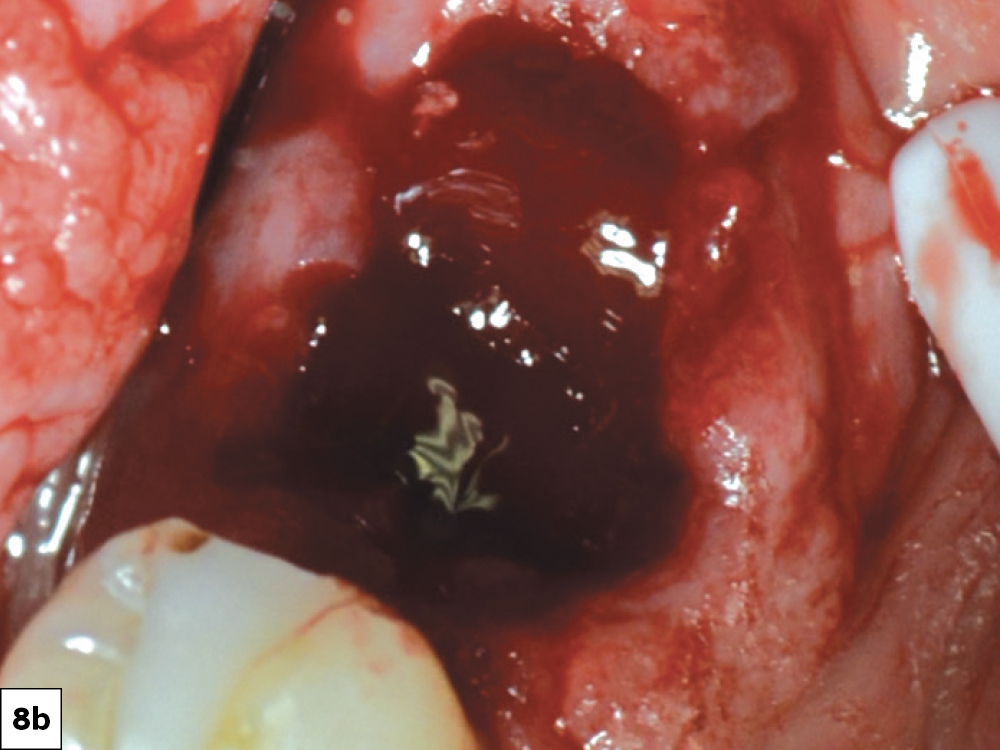
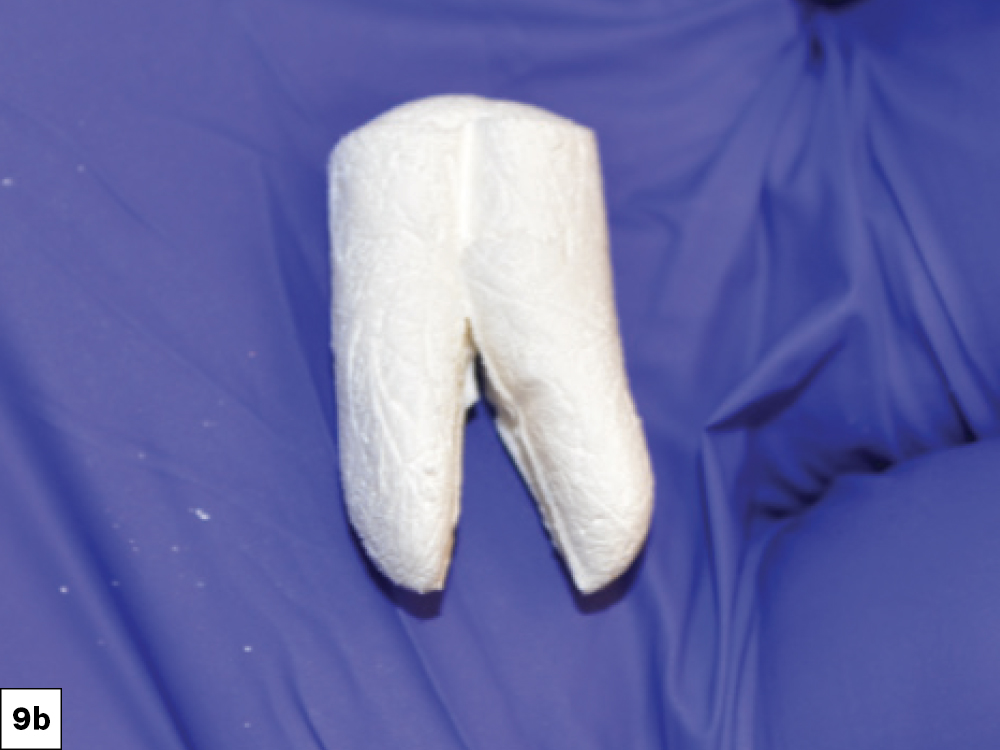
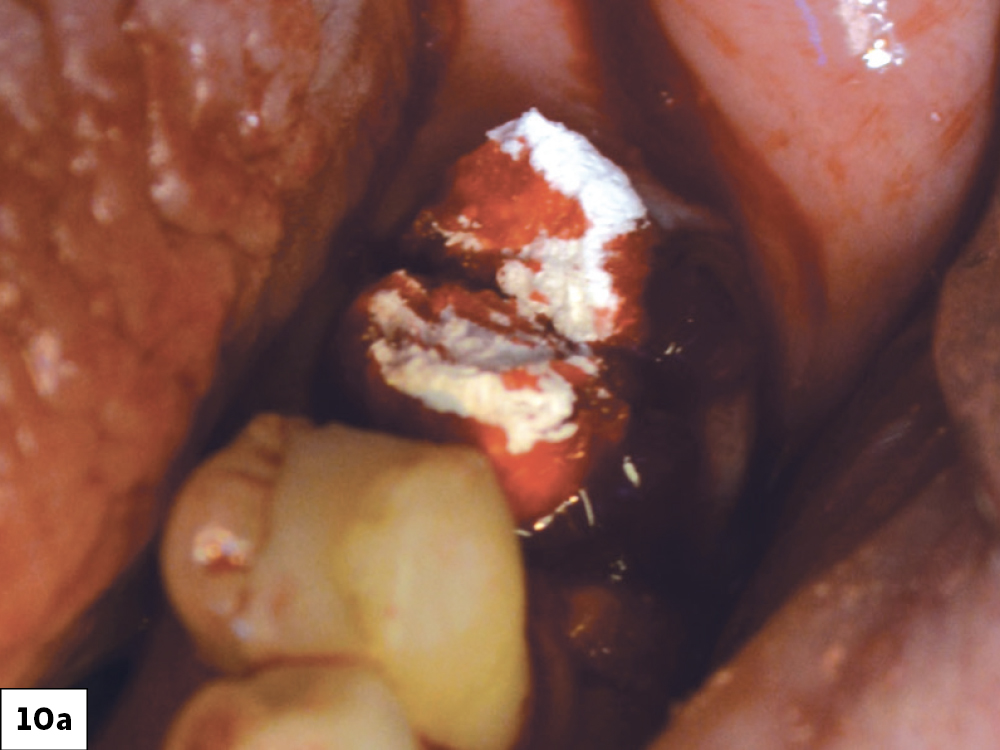
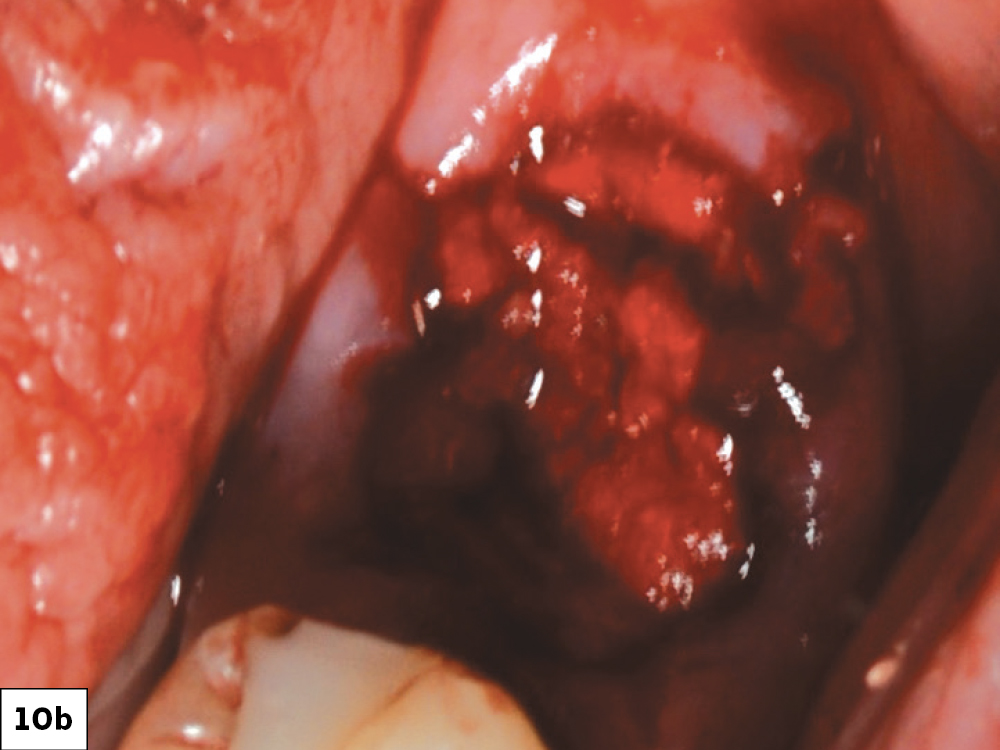
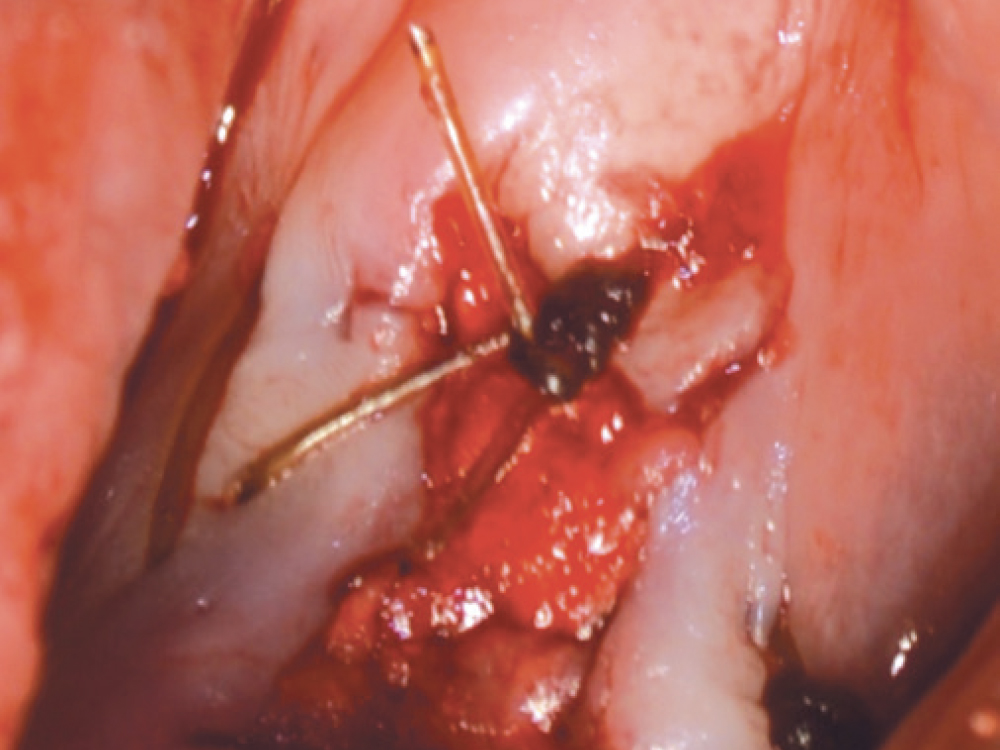
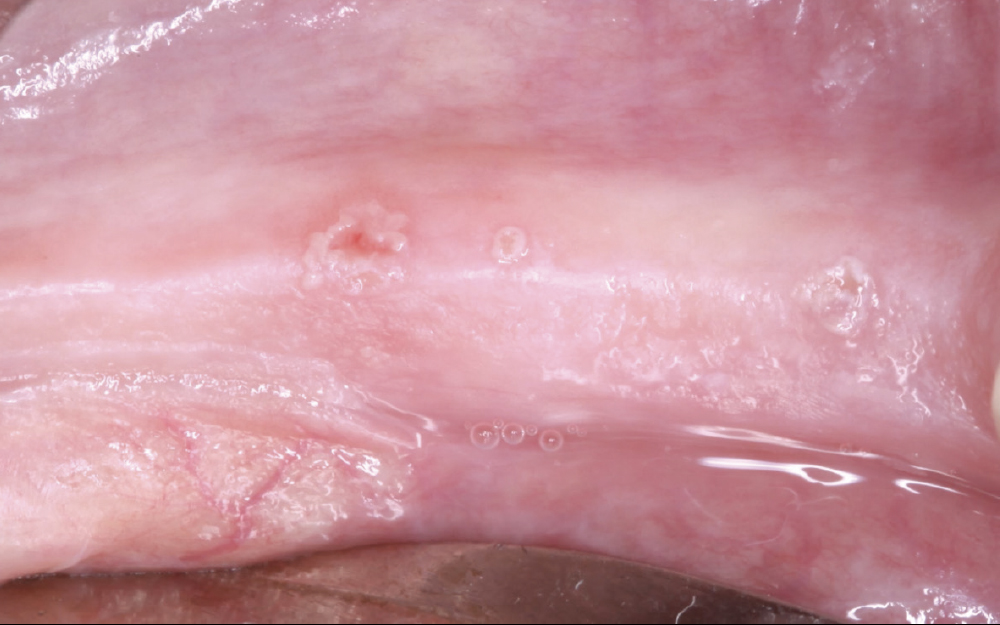
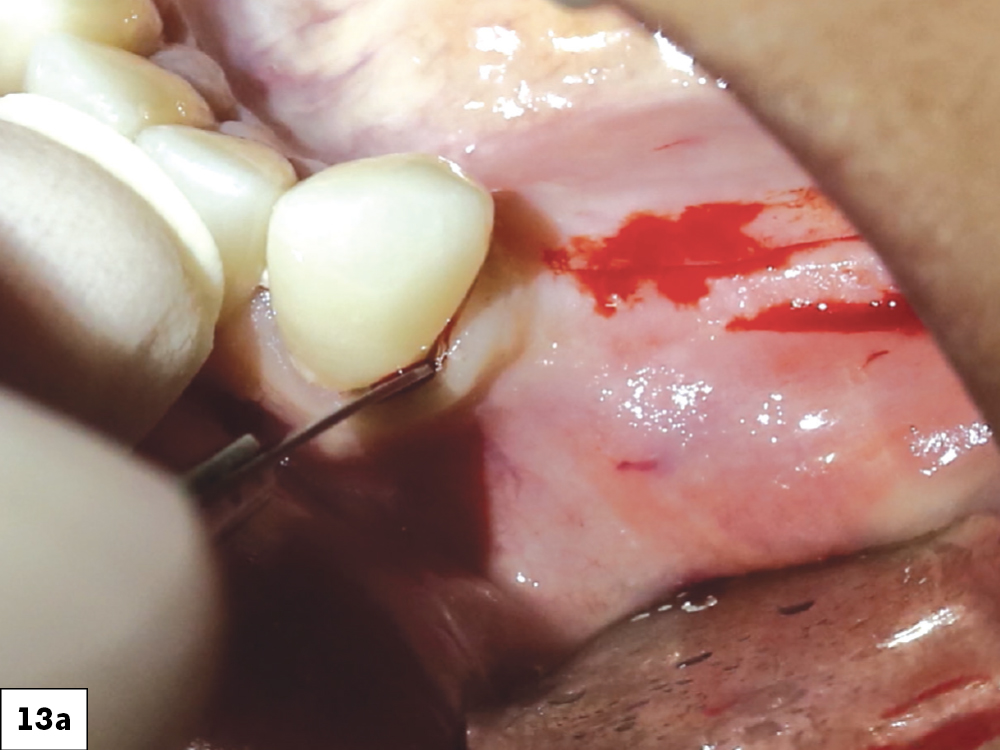
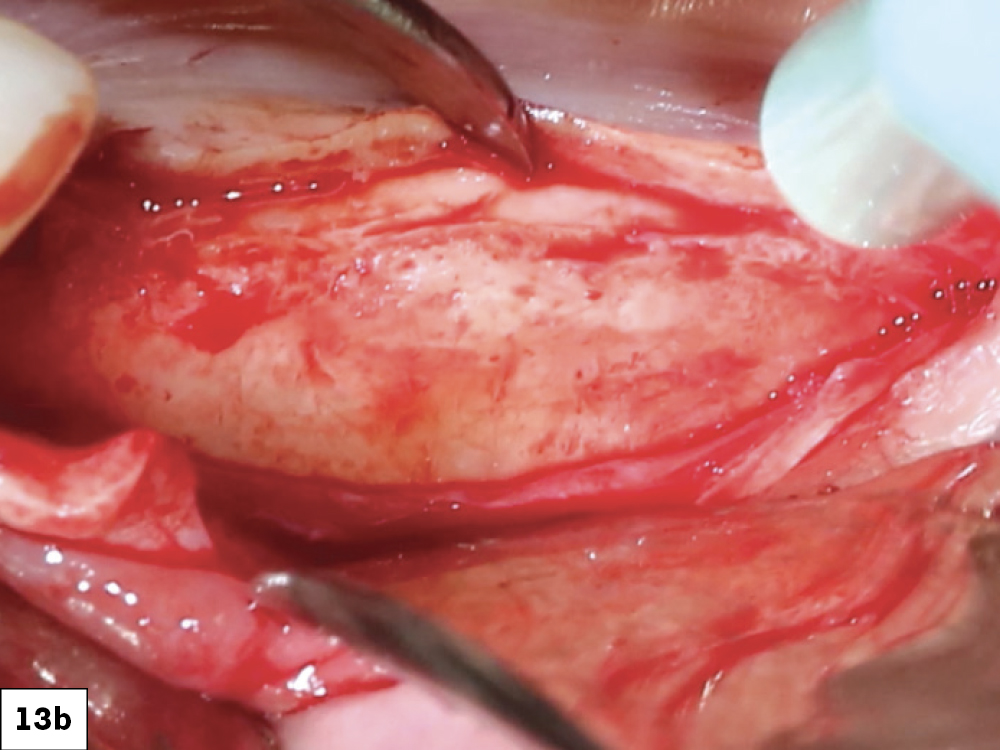
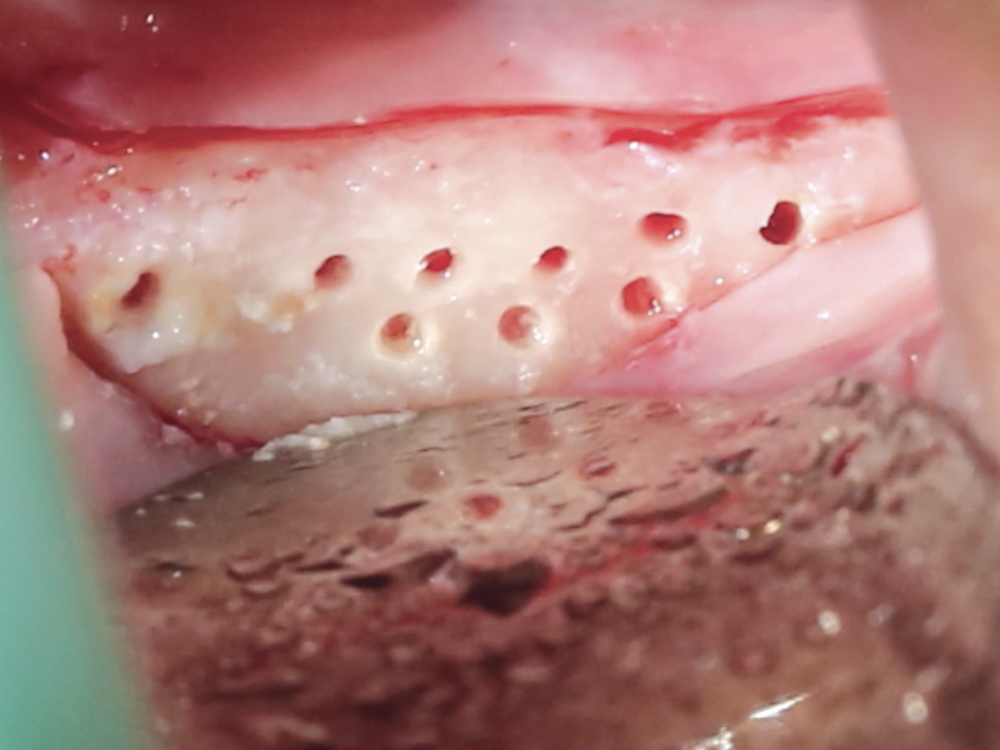
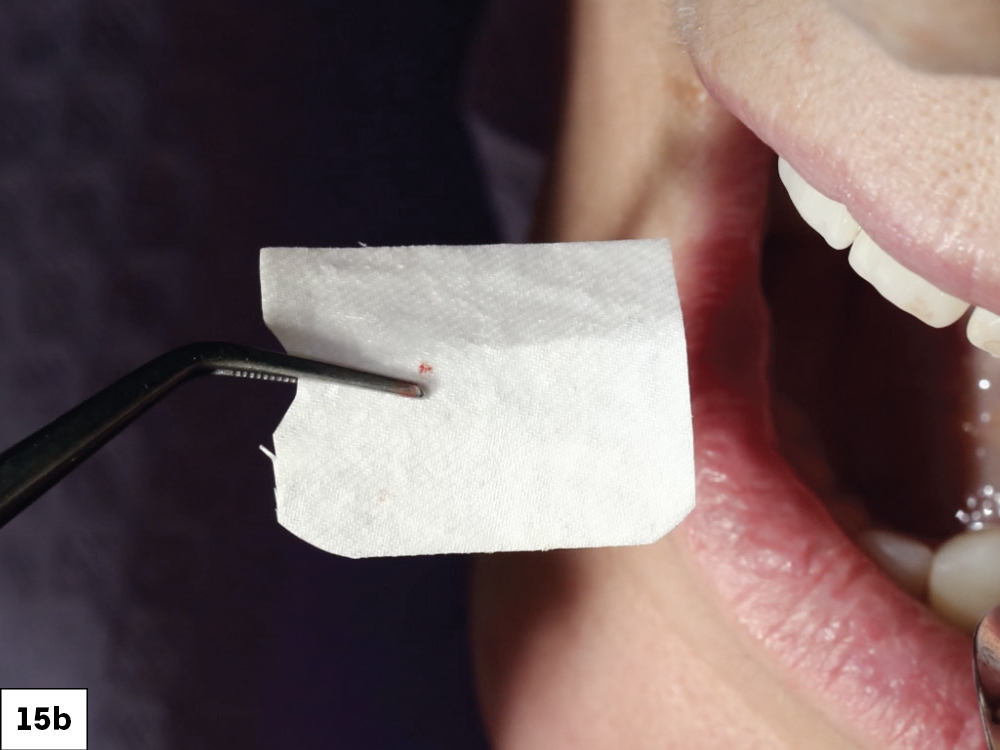
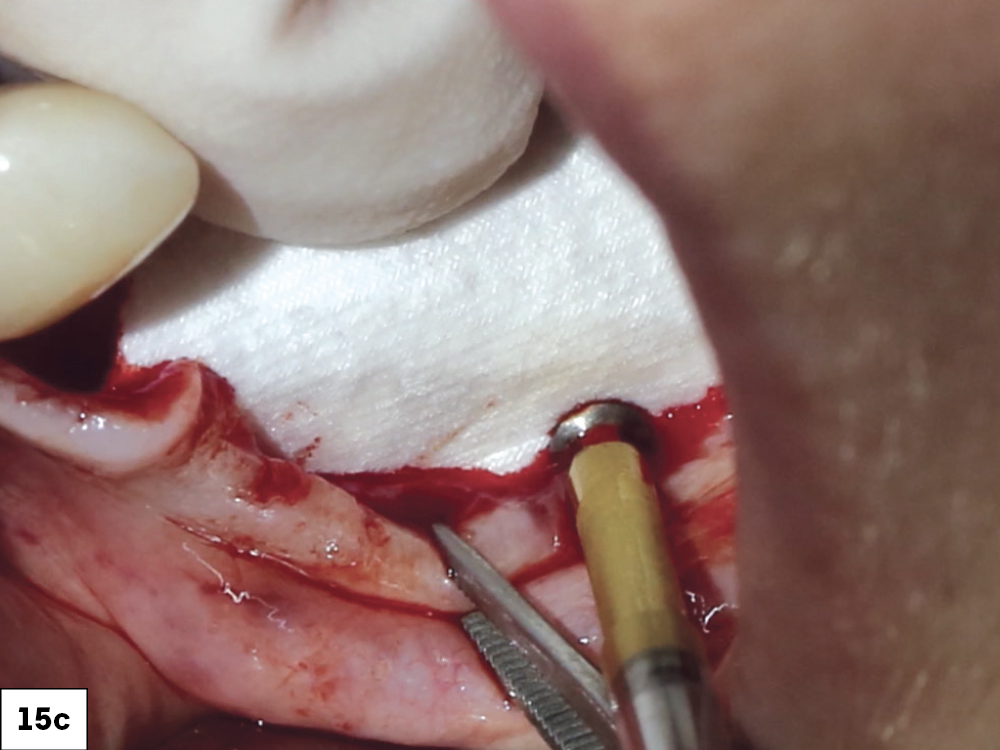
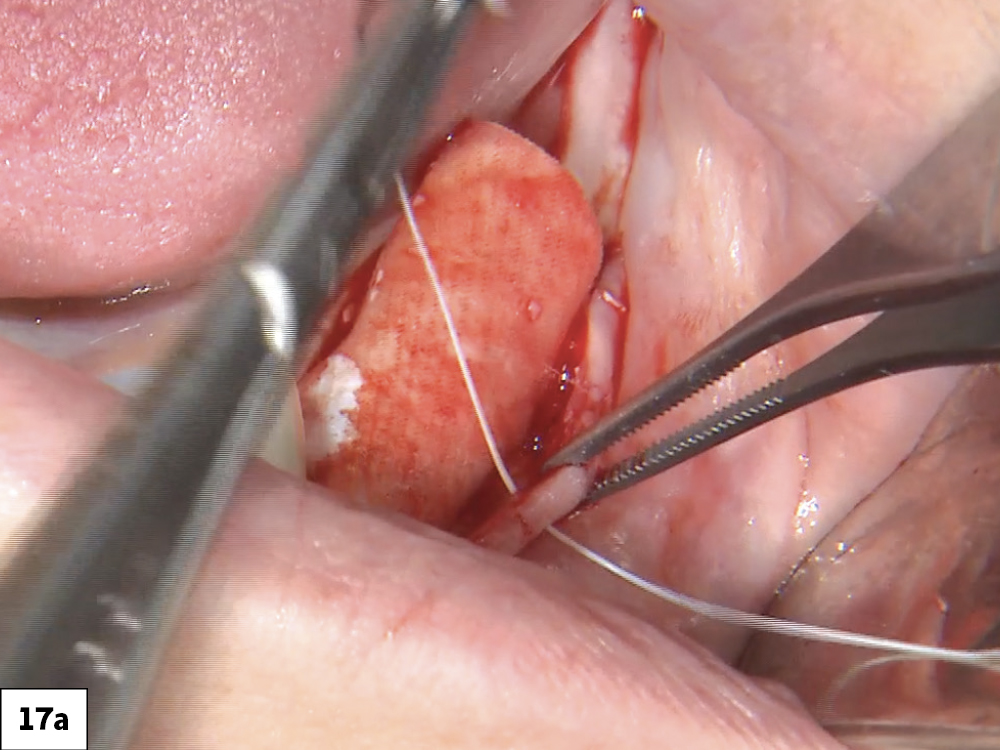
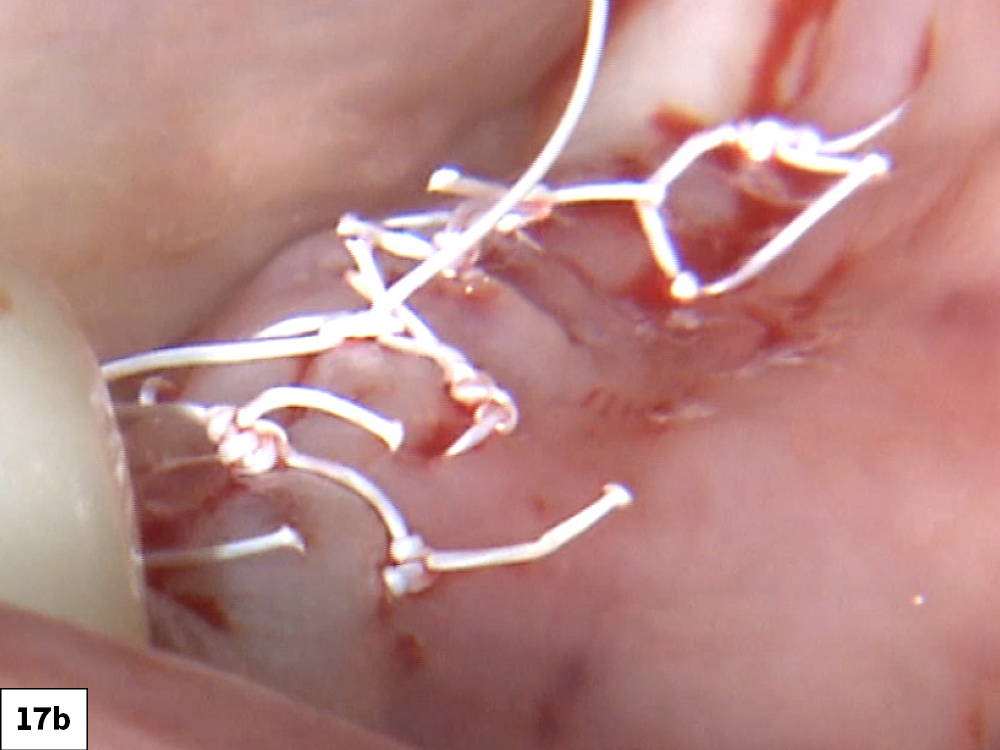
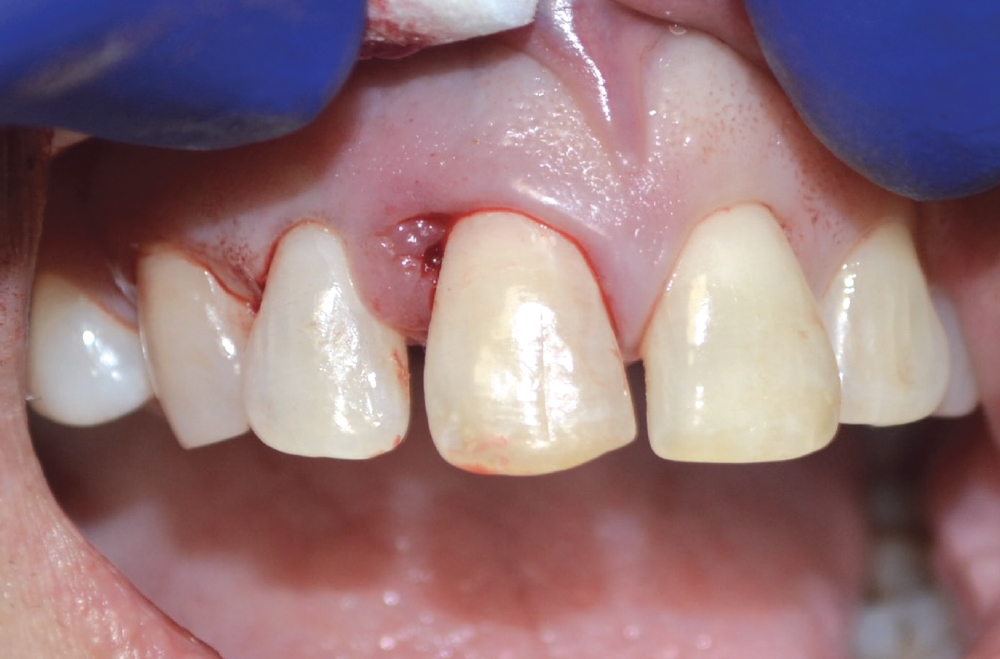
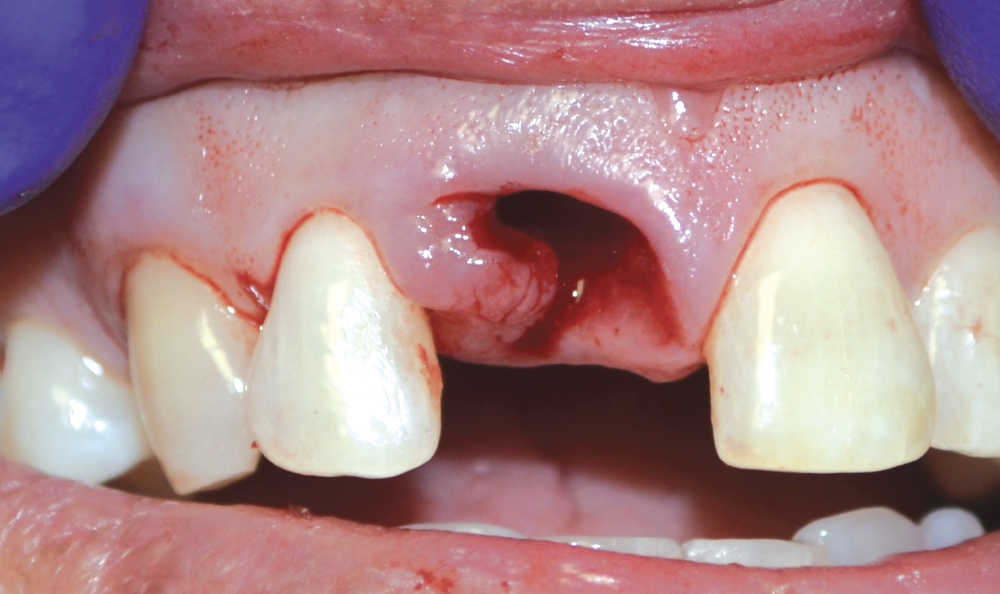
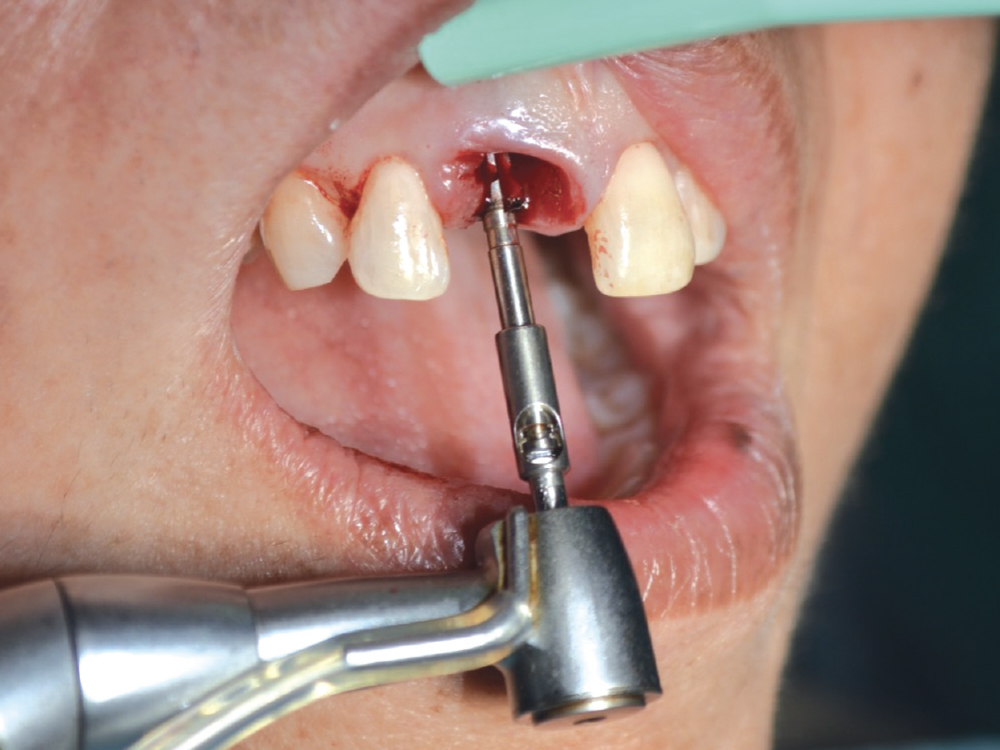
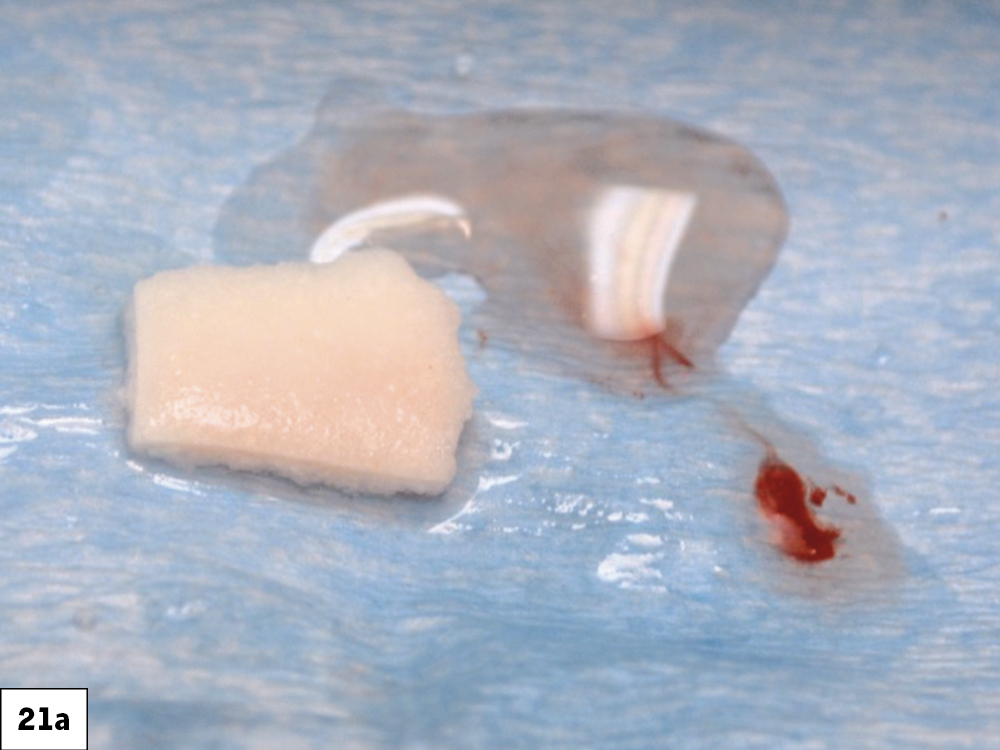
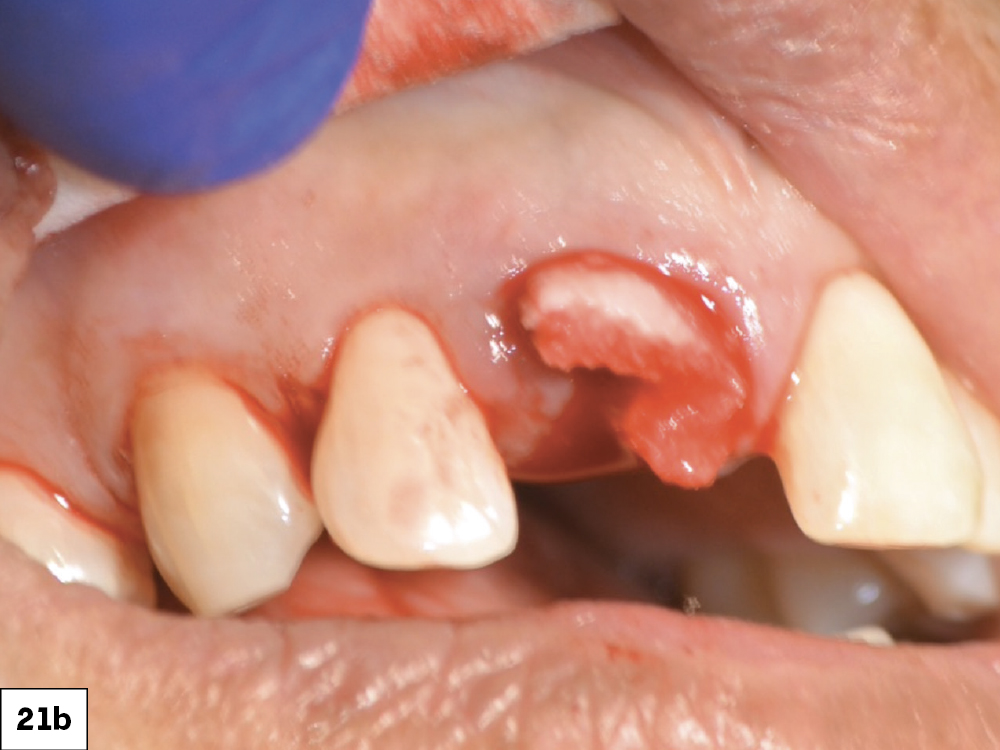
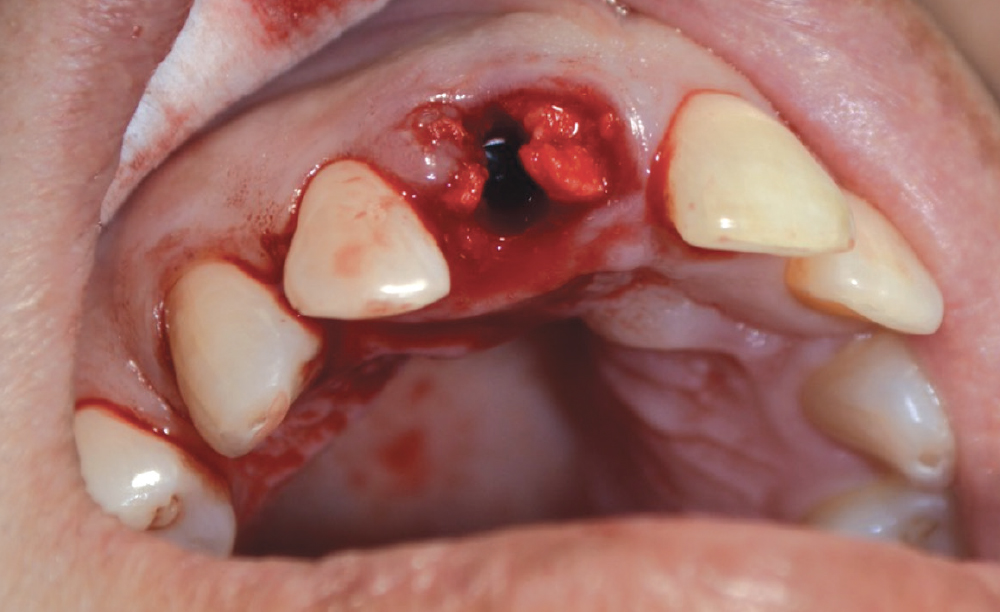
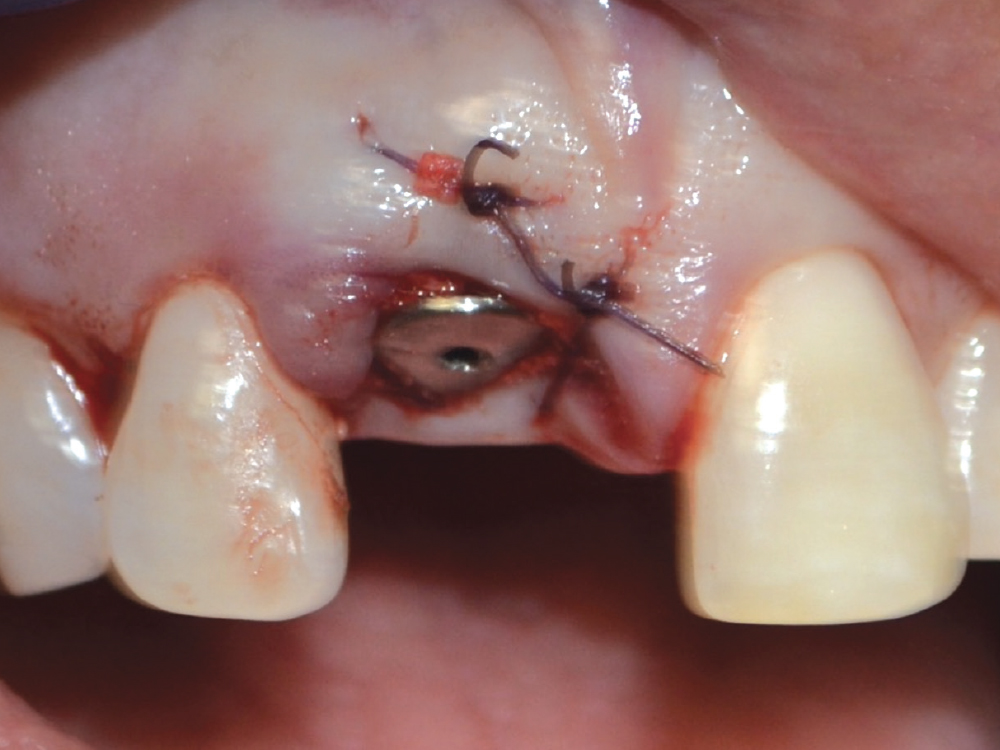
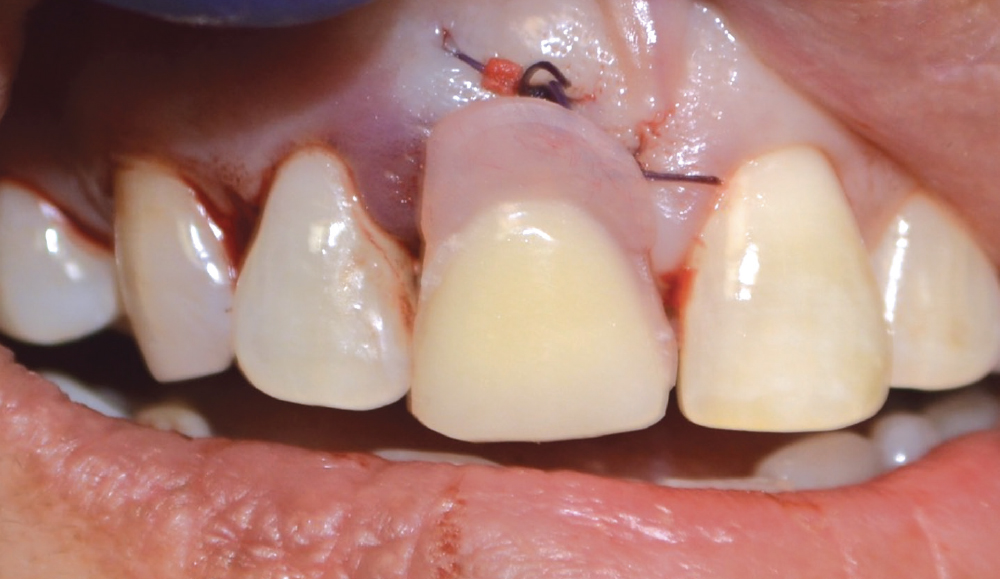
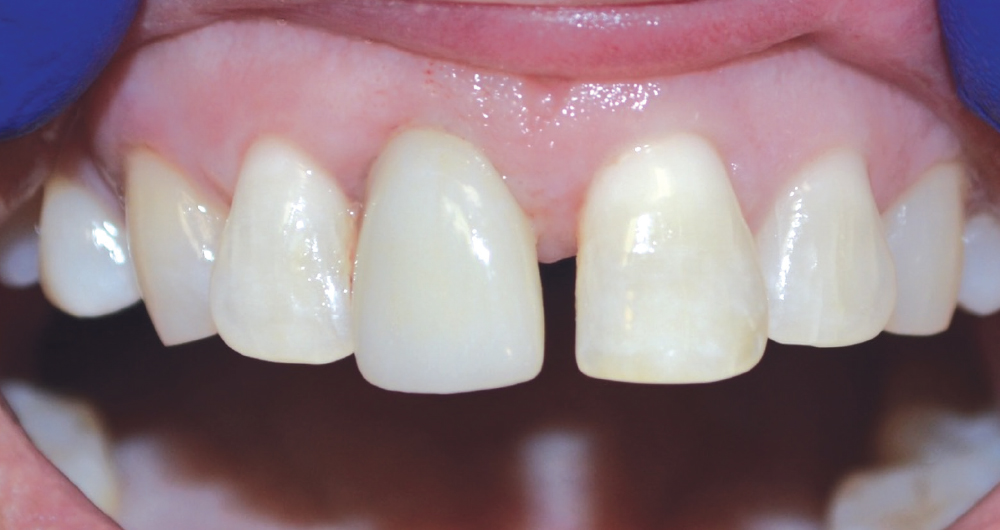
CASE ONE
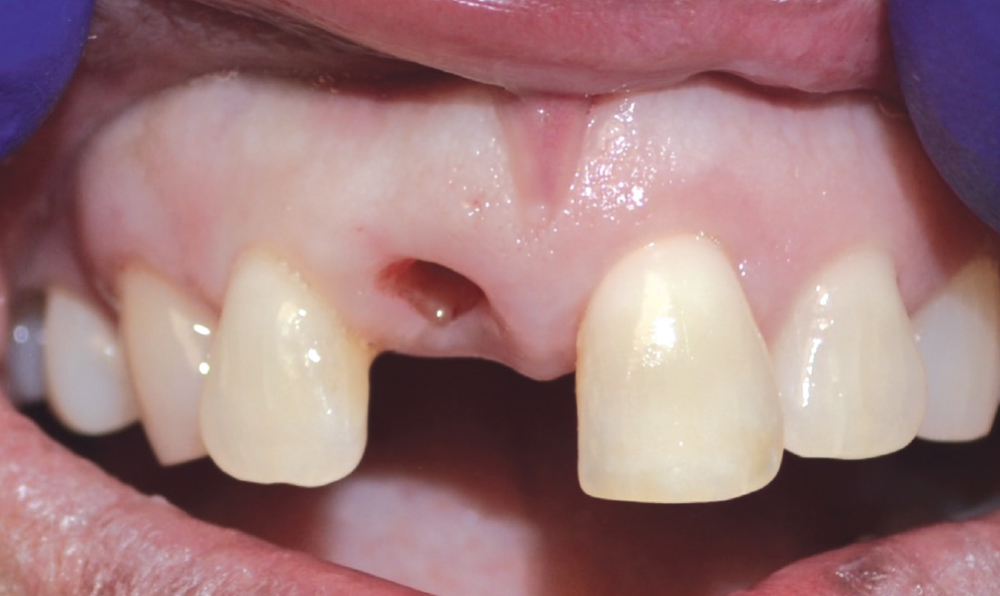
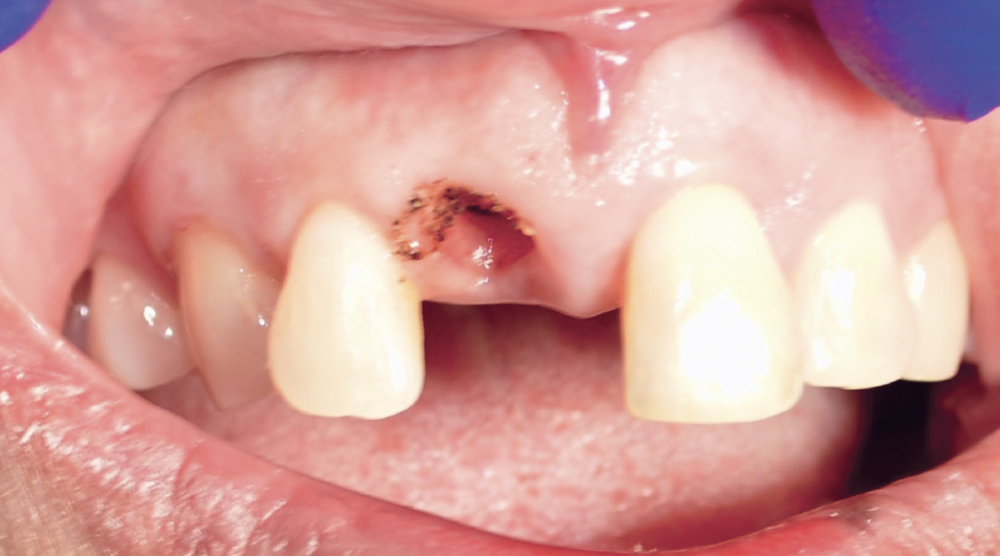
CASE TWO
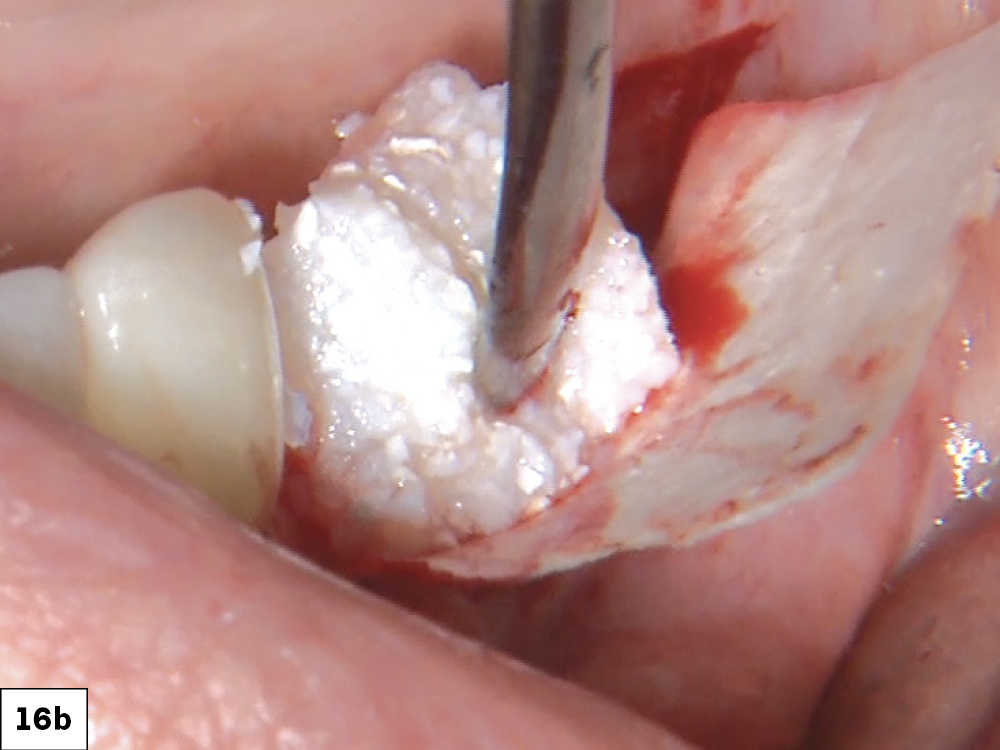
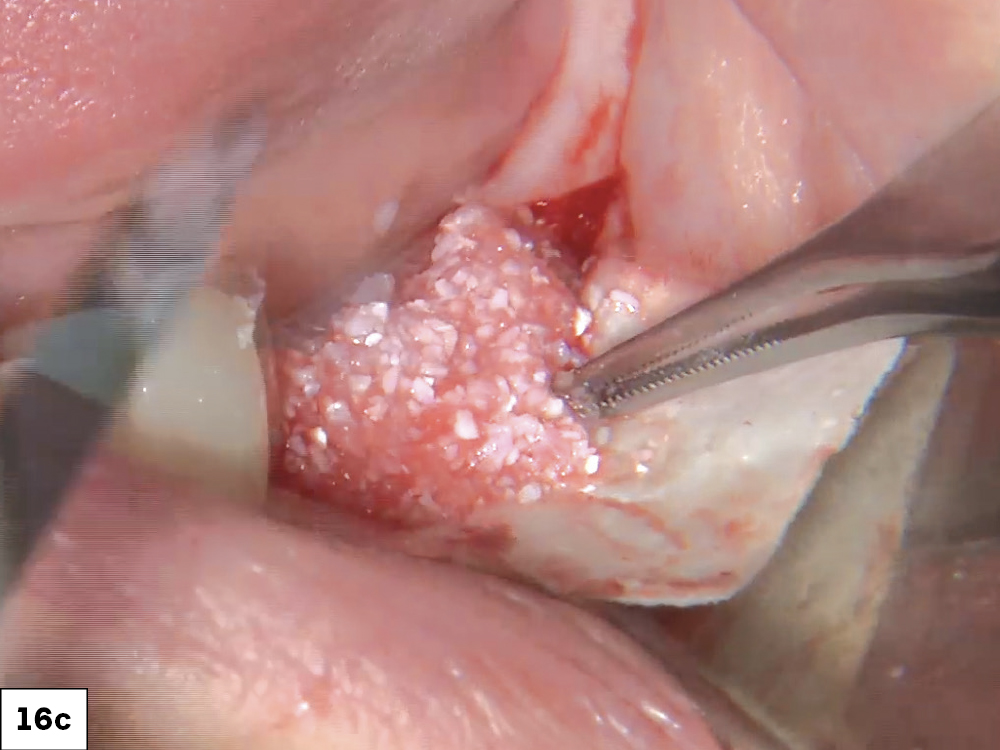
As opposed to the intact socket depicted above, a socket with a 1-, 2- or 3-wall defect needs to be handled a bit differently. Although the graft material choices are the same, the inclusion of a membrane becomes necessary for success. The membrane can be either a resorbable collagen or polyglycolic acid (PGA) or non-resorbable such as PTFE. It is crucial to not only confine the material, but also to create a barrier resistant to soft-tissue ingrowth.
LATERAL AND VERTICAL DEFECTS
In this type of clinical situation, one must not only provide a framework for new bone growth, but also resist movement and soft-tissue compression on the graft. For these types of defects, materials should be used that take longer to resorb or hold their space more efficiently. Membranes are mandatory. To resist compressive forces, a reinforcement structure — such as titanium mesh, a titanium reinforced PTFE membrane, or tenting screws — can be added.
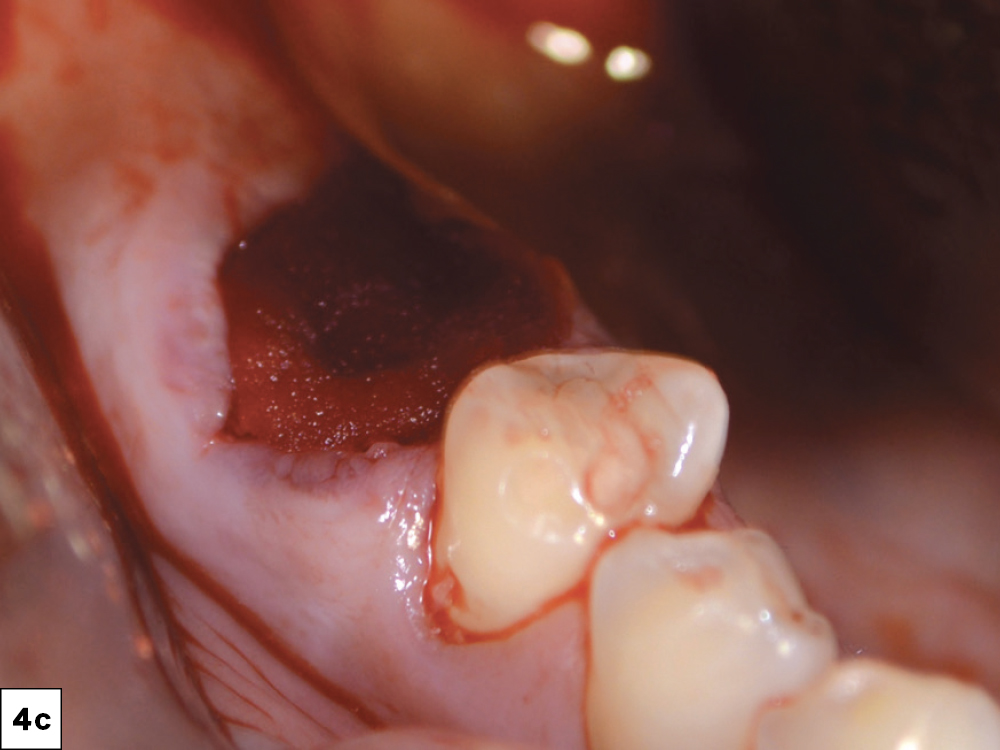
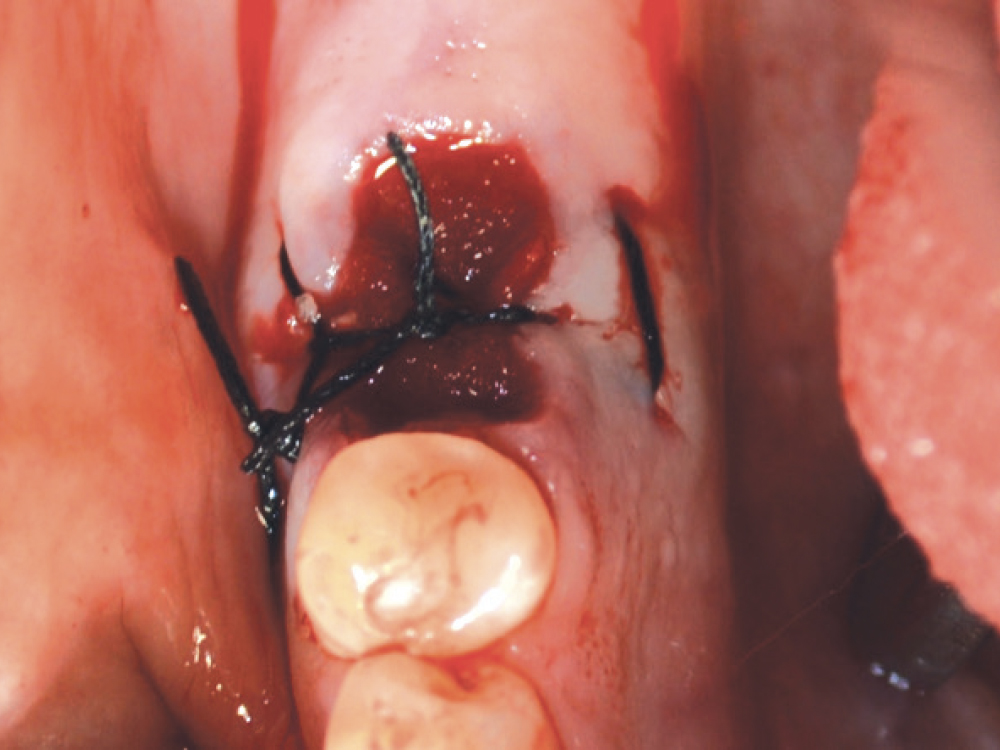
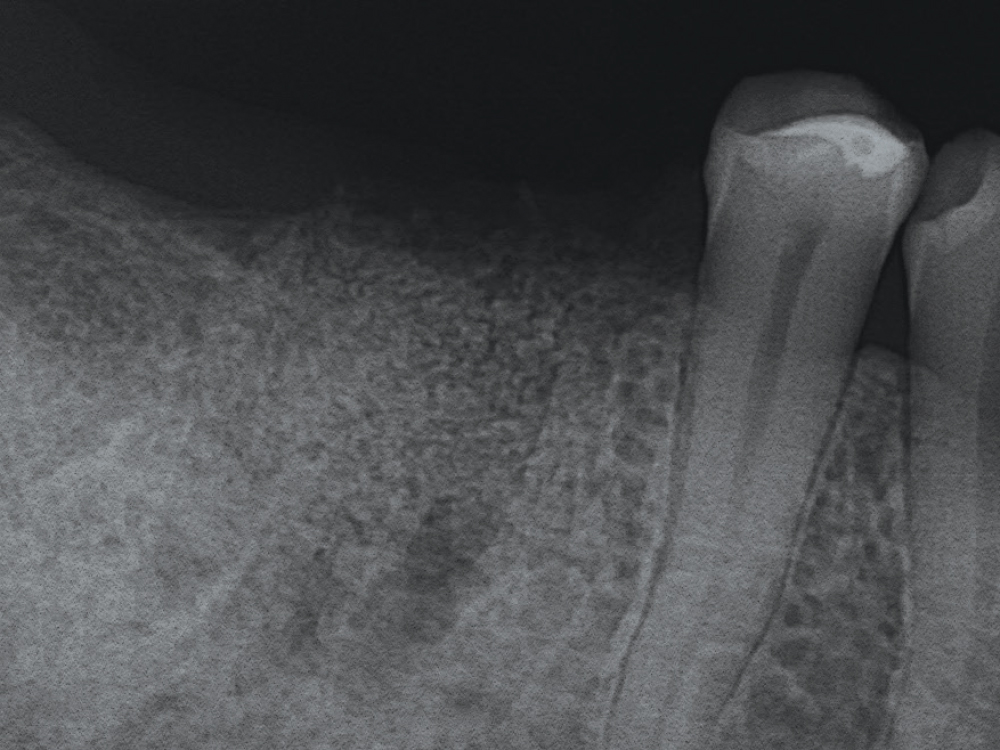
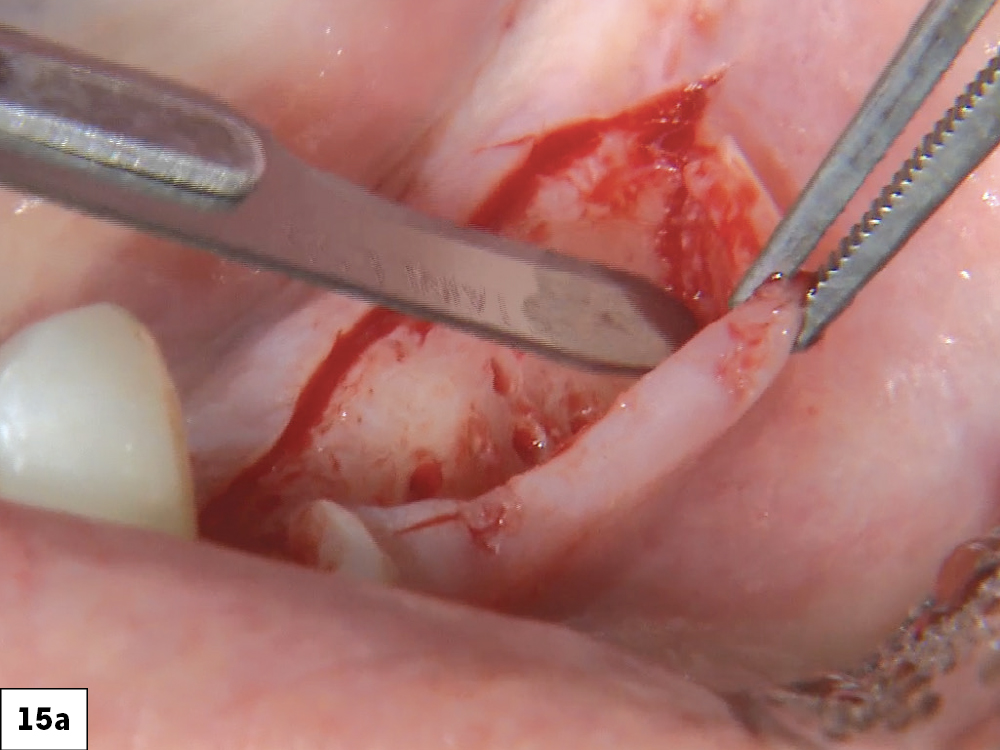
CASE THREE
GRAFTING THE “GAP” IN IMPLANT PLACEMENT
When placing an implant, specifically when it is an immediate placement in conjunction with an extraction, you are left with a gap between the implant body and the alveolus. Although this gap has the ability to fill in on its own, it does not do so predictably. Therefore, grafting this space is a way to ensure better and more predictable bone fill-in results. Since it is an infrabony defect, the same materials used for socket preservation can be utilized with the addition of a membrane.
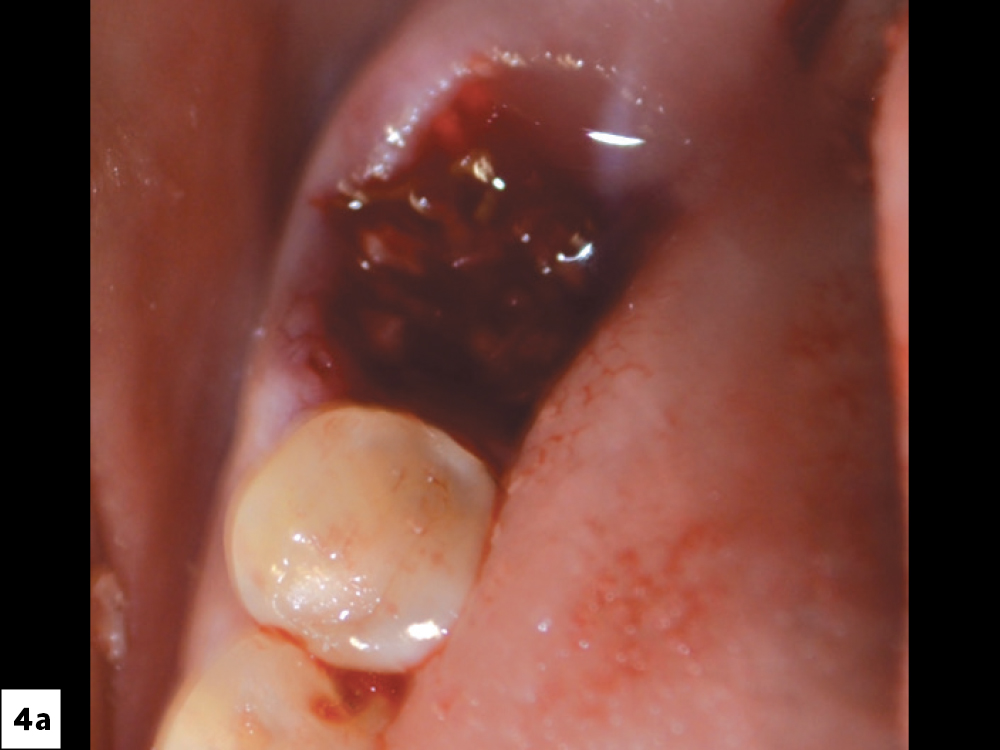
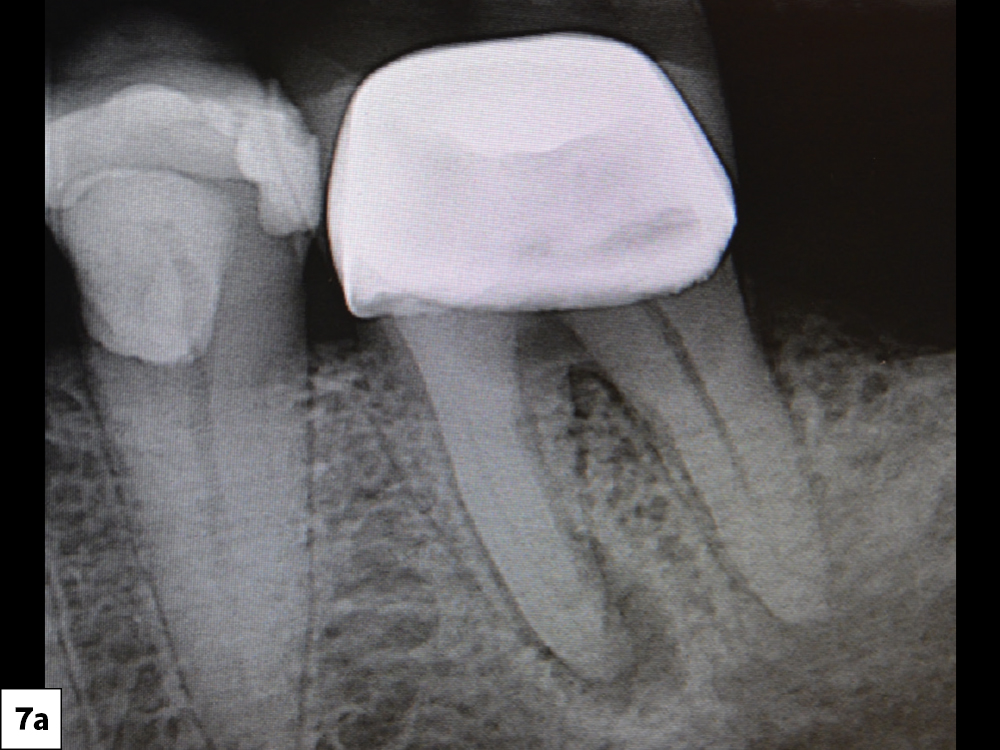
CASE FOUR
CONCLUSION
Through the examples listed in this article, I present the materials and techniques that I have developed and that work predictably for me. This is, by no means, the only way to do these procedures. Your success depends on your ability to evaluate the conditions presented to you clinically, your surgical skill level and finally your level of experience.
All third-party trademarks are property of their respective owners.
References
-
Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8:114-124.
-
Resnik R. Bone substitutes in oral implantology. Chairside® magazine. 2018;12(3)83-92.